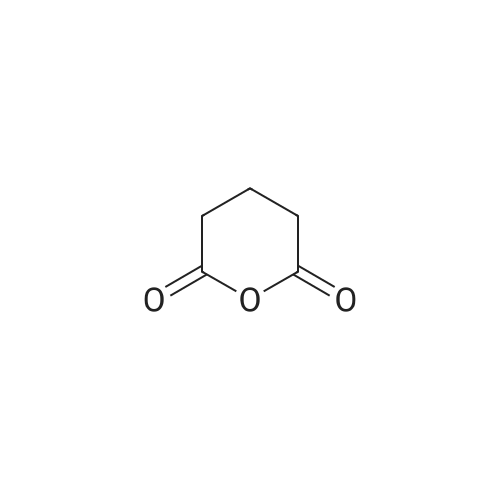
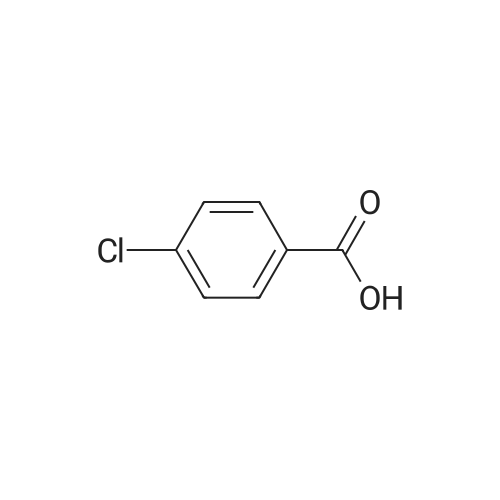
| 11% |
With dmap; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride In dichloromethane at 45℃; |
3.4. General Synthetic Methods for Compounds 2-16
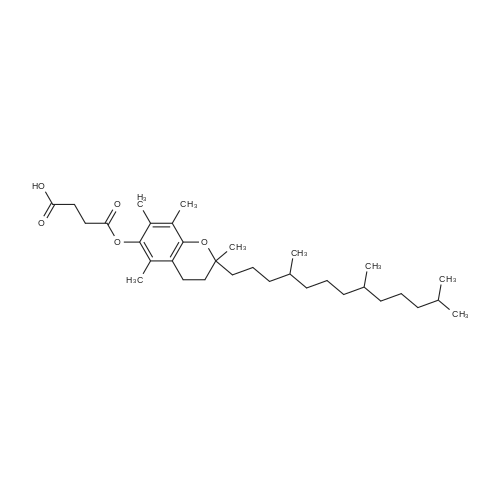
General procedure: Benzoic acid-derived reagent (1-2 equivalent) was added to a solution of 1 (50.0 mg,0.18 mmol), DMAP (21.8 mg, 0.18 mmol), and EDCl (110.7 mg, 0.71 mmol) in 15 mL drydichloromethane (DCM). The reaction mixture was stirred at 45 °C, and the progress ofthe reaction was monitored by silica gel TLC and UPLC-MS. After 1-3 h, the reactionmixture was quenched with water and diluted with DCM. The organic layer was separated,and the solvent was removed under reduced pressure. The residue was purified bysilica gel CC followed by semi-preparative HPLC to yield unreacted 1 and derivatives 2-16 (Figures S4-S48).Brefeldin A 7-O-benzoate (2): Known compound. Colorless oil; yield 11%; 1H NMR(400 MHz, CDCl3) δ 8.03-7.98 (2H, overlapped), 7.56 (1H, m), 7.47-7.43 (2H, overlapped),7.37 (1H, dd, J = 15.7, 3.1 Hz), 5.94 (1H, dd, J = 15.7, 1.9 Hz), 5.73 (1H, m), 5.40 (1H, m), 5.26(1H, dd, J = 15.2, 9.0 Hz), 4.86 (1H, m), 4.17 (1H, m), 2.53-2.33 (3H, overlapped), 2.06-1.96(2H, overlapped), 1.92 (1H, m), 1.88-1.80 (2H, overlapped), 1.77-1.70 (2H, overlapped),1.53 (1H, m), 1.26 (3H, d, J = 6.2 Hz), 0.96 (1H, m); 13C NMR (100 MHz, CDCl3) δ 166.3 (C =O), 166.3 (C = O), 151.6 (CH), 136.1 (CH), 133.1 (CH), 131.1 (CH), 130.6 (C), 129.7 (CH × 2),128.5 (CH × 2), 117.9 (CH), 76.1 (CH), 76.0 (CH), 71.9 (CH), 52.6 (CH), 44.1 (CH), 40.3(CH2), 38.9 (CH2), 34.2 (CH2), 32.0 (CH2), 26.8 (CH2), 21.0 (CH3). ESIMS m/z 407.4 [M +Na]+. |
| 11% |
With dmap; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride In dichloromethane at 45℃; |
3.4. General Synthetic Methods for Compounds 2-16
General procedure: Benzoic acid-derived reagent (1-2 equivalent) was added to a solution of 1 (50.0 mg,0.18 mmol), DMAP (21.8 mg, 0.18 mmol), and EDCl (110.7 mg, 0.71 mmol) in 15 mL drydichloromethane (DCM). The reaction mixture was stirred at 45 °C, and the progress ofthe reaction was monitored by silica gel TLC and UPLC-MS. After 1-3 h, the reactionmixture was quenched with water and diluted with DCM. The organic layer was separated,and the solvent was removed under reduced pressure. The residue was purified bysilica gel CC followed by semi-preparative HPLC to yield unreacted 1 and derivatives 2-16 (Figures S4-S48).Brefeldin A 7-O-benzoate (2): Known compound. Colorless oil; yield 11%; 1H NMR(400 MHz, CDCl3) δ 8.03-7.98 (2H, overlapped), 7.56 (1H, m), 7.47-7.43 (2H, overlapped),7.37 (1H, dd, J = 15.7, 3.1 Hz), 5.94 (1H, dd, J = 15.7, 1.9 Hz), 5.73 (1H, m), 5.40 (1H, m), 5.26(1H, dd, J = 15.2, 9.0 Hz), 4.86 (1H, m), 4.17 (1H, m), 2.53-2.33 (3H, overlapped), 2.06-1.96(2H, overlapped), 1.92 (1H, m), 1.88-1.80 (2H, overlapped), 1.77-1.70 (2H, overlapped),1.53 (1H, m), 1.26 (3H, d, J = 6.2 Hz), 0.96 (1H, m); 13C NMR (100 MHz, CDCl3) δ 166.3 (C =O), 166.3 (C = O), 151.6 (CH), 136.1 (CH), 133.1 (CH), 131.1 (CH), 130.6 (C), 129.7 (CH × 2),128.5 (CH × 2), 117.9 (CH), 76.1 (CH), 76.0 (CH), 71.9 (CH), 52.6 (CH), 44.1 (CH), 40.3(CH2), 38.9 (CH2), 34.2 (CH2), 32.0 (CH2), 26.8 (CH2), 21.0 (CH3). ESIMS m/z 407.4 [M +Na]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +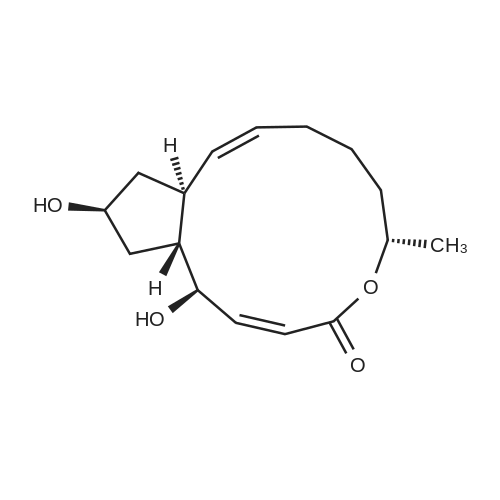

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping