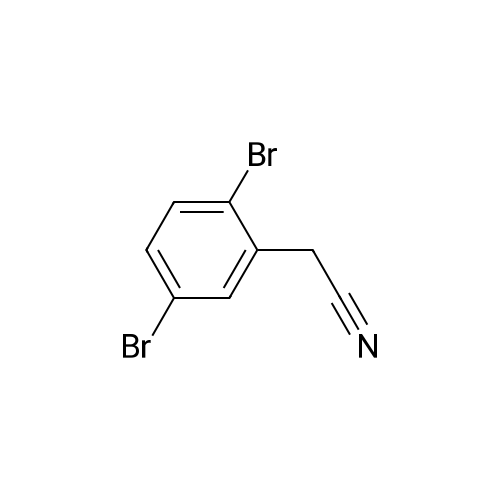
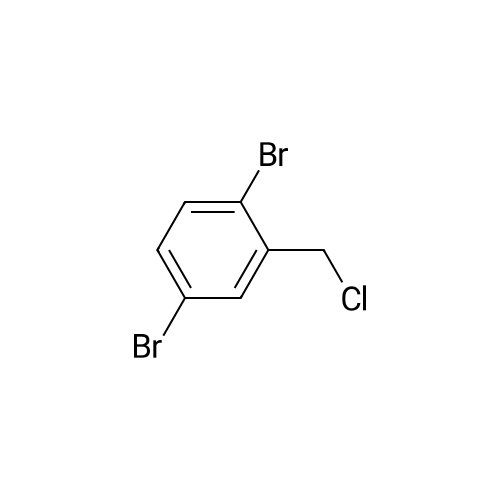
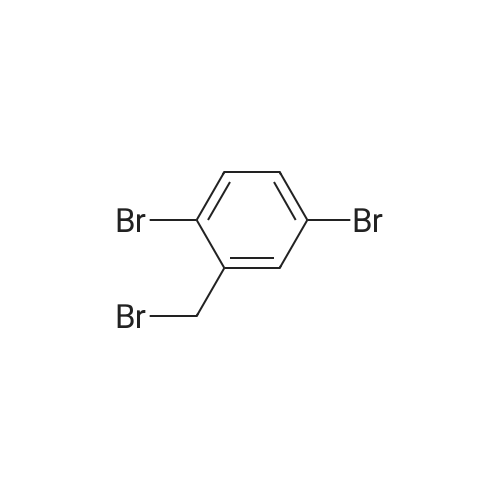
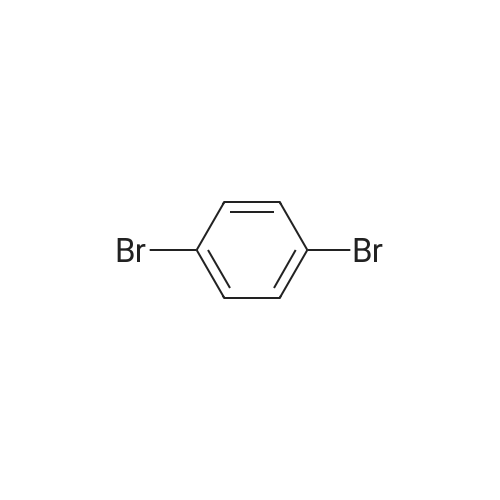
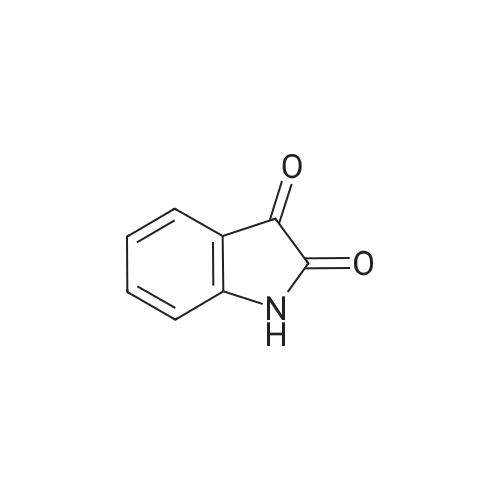
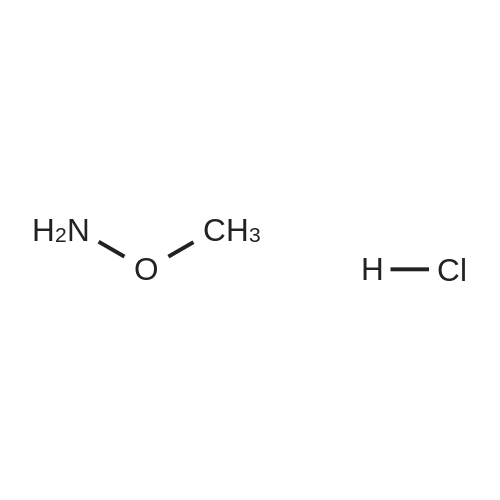
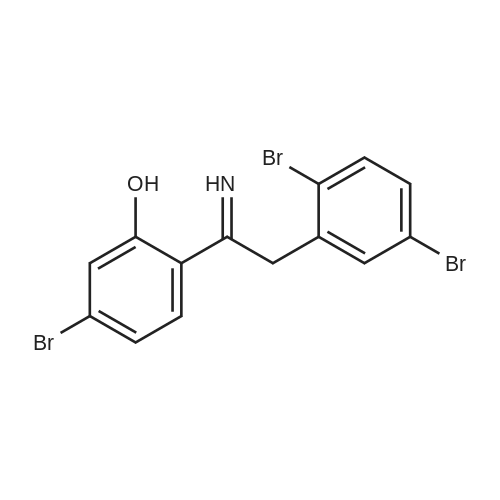
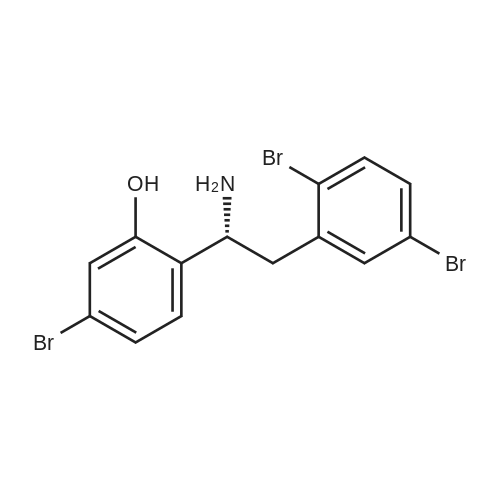
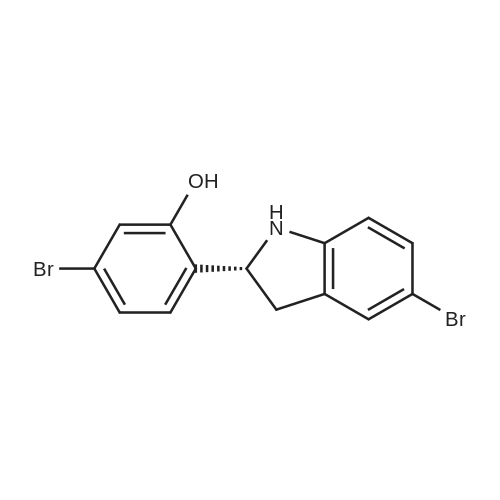
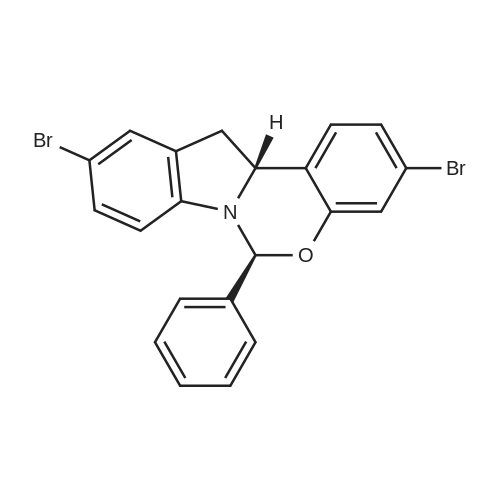
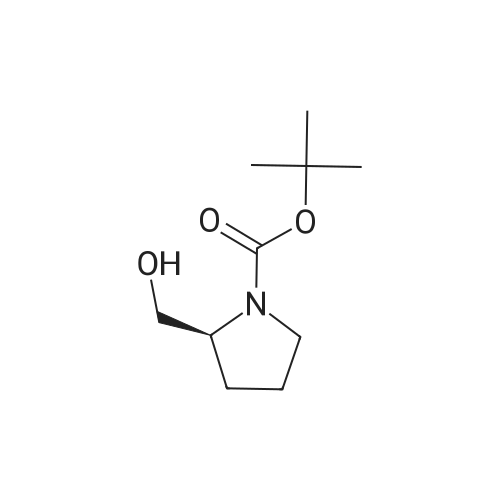
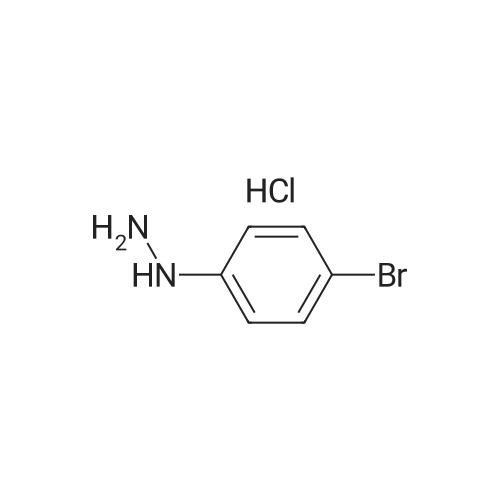
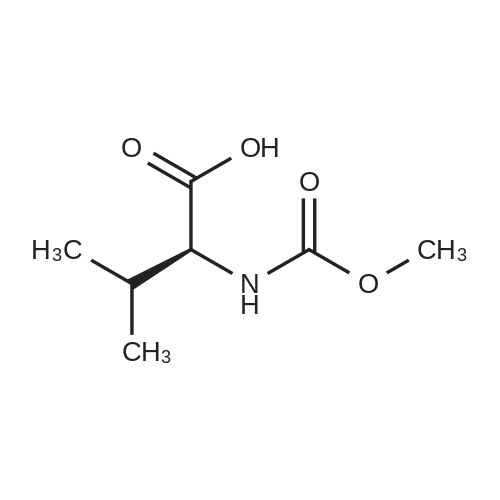
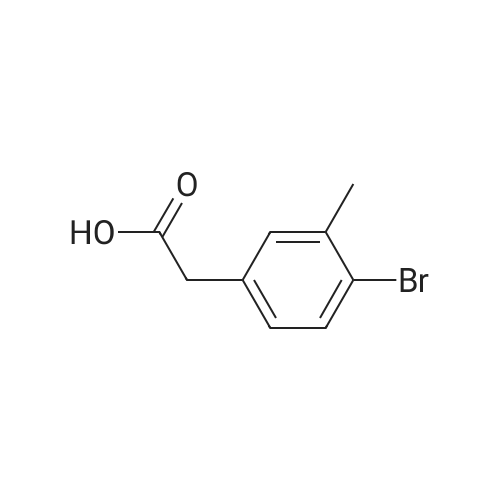
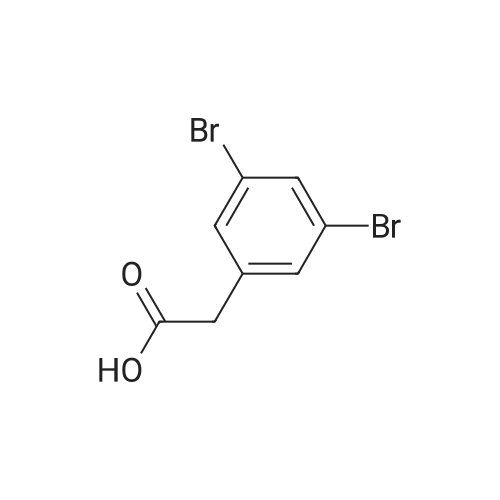
|
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 20℃; for 1h;Inert atmosphere; |
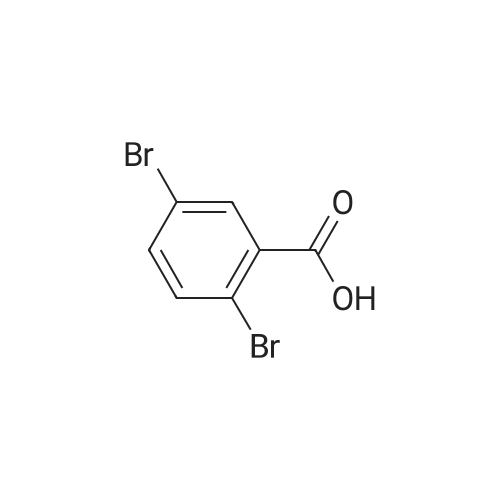
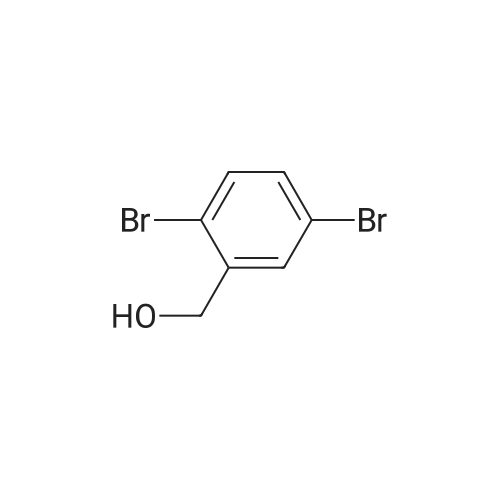
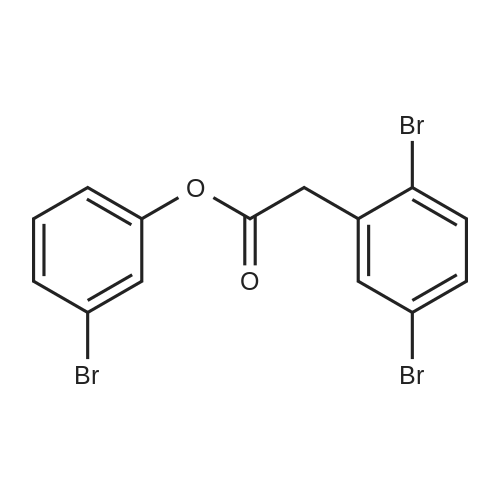
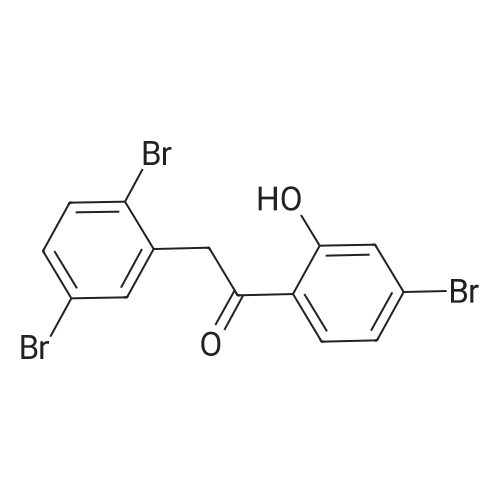
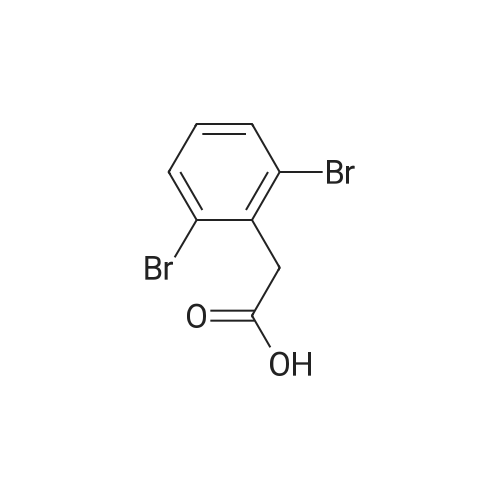
A 2 L 3 -neck round-bottomed flask equipped with overhead stirring and a nitrogen inlet was charged with acid 1 (100 g, 339 mmol) and dichloromethane (1200 mL, 12 vol) at ambient temperature. After all solids were dissolved, N,N-dimethylformamide (1.24 g, 0.05 equiv) was added followed by slow addition of oxalyl chloride (48.3 g, 1.1 equiv) over 20 minutes at ambient temperature. The resulting mixture was stirred under nitrogen at ambient temperature for 1 hour and monitored by HPLC for complete conversion. After the reaction was complete, batch volume was concentrated to 500 mL. A separate 2 L 3 -neck round-bottomed flask equipped with overhead stirring and a nitrogen inlet was charged with acid 3-bromophenol (62.9 g, 356 mmol, 1.05 equiv) and dichloromethane (530 mL, 5 vol). 2,6-Lutidine (73.5g, 2.0 equiv) was charged maintaining temperature below 25C, and the resulting mixture was cooled to 0-5C. The solution of acyl chloride in dichloromethane generated above was charged slowly while maintaining batch temperature at between 0 and 5C. The resulting mixture was stirred for 1 hour and monitored by HPLC for complete conversion. After the reaction was complete, the batch was quenched with 1N HCl solution (530 ml). The aqueous layer was cut, and the organics were washed with water (530 ml). The volume of organics was then reduced to 300 mL and dichloromethane was replaced with acetonitrile via continuous distillation. During the solvent swap, the solution of 2 becomes a slurry. At this time, water (318 ml) was charged slowly over 30 minutes, and the resulting slurry was stirred for 60 minutes. The slurry was then filtered and washed with 50% acetonitrile/water (318 ml). After drying under vacuum and nitrogen sweeping, 146.0 g (91 %> yield) of the title compound was obtained as a white crystalline solid. 1H NMR (CDCl3, 400 MHz): delta = 7.54 (d, J = 3.0 Hz, 1H), 7.49 (d, J= 10.6 Hz, 1H), 7.39 (dd, J= 10.0, 2.4 Hz, 1H), 7.35-7.32 (m, 2H), 7.25 (d, J= 10.1 Hz, 1H), 7.10 (dd, J= 10.6, 1.6 Hz, 1H), 4.00 (s, 2H). |
|
|
Compound PA 6016 -B 1 (100 g, 339 mmol) was dissolved in DCM (1200 mL) and oxalyl chloride (48.3 g, 380.6 mmol) was added slowly over 20 min followed by the slow addition of DMF , 17.0 mmol) for 1 h at room temperature.After the reaction was completed, the reaction solution was concentrated to 500 mL to give an acid chloride solution.To another 2000 mL three-neck reaction flask was added m-bromophenol (62.9 g, 356 mmol) DCM (530 mL) and 2,6-lutidine, cooled to 0_5 C,Slowly add the above-obtained acid chloride solution, dropping temperature was controlled at 0-5 C,After the addition was completed 0-5 C stirred reaction 1h.IN HC1 (530 mL) was slowly added dropwise after the reaction was completed.The organic layer was washed with water (530 mL) after layering, spin-dried and then added with 300 mL of acetonitrile and stirred well.Slowly add 318 mL of water at room temperature, a large number of precipitation, filtration,The filter cake was washed with 318 mL of acetonitrile / water (1: 1) and dried to give a white product (146.0 g)Yield 91% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping