| 90% |
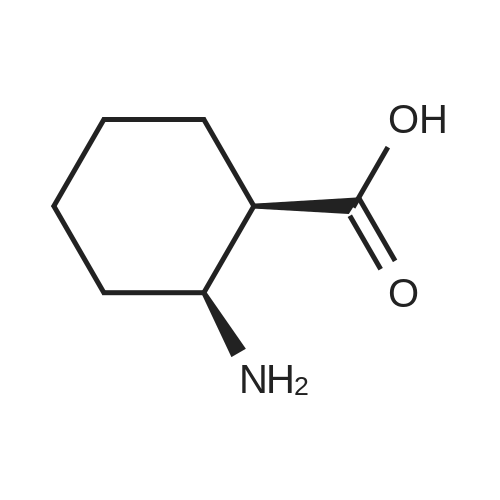
Stage #1: (2S,5R)-1-[(1,1-dimethylethyl)carbonyl]-5-[(benzyloxy)amino]piperidine-2-carboxylic acid With triethylamine; isobutyl chloroformate In tetrahydrofuran at -20℃; for 0.25h;
Stage #2: (3aR,7aS)-2-hydroxyhexahydro-1H-isoindol-1,3(2H)-dione In tetrahydrofuran at -20 - 20℃; for 1h; |
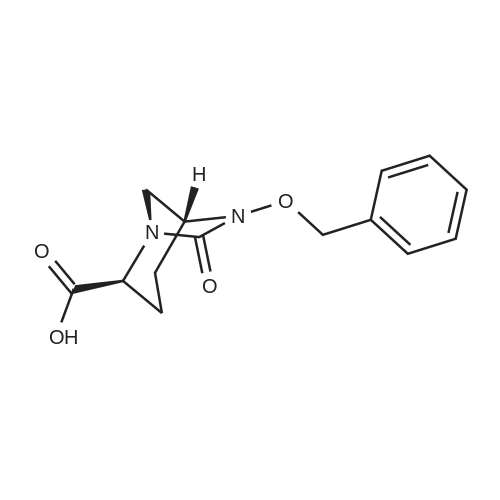
61 Example 61(2S, 5R) -5 - ((benzyloxy) amino) piperidine-1,2-dicarboxylic acid 1-tert-butyl ester 2 - ((3aR, 7aS) -1,3- dioxo-hexahydro -1H- isoindol -2 (3H) - yl) ester (II-33)
(2S, 5R) -5 - ((benzyloxy) amino) -1- (tert-butoxycarbonyl) piperidine-2-carboxylic acid (Reference Example 4,3.504g, 10mmol) was dissolved in dehydrated tetrahydrofuran (50 mL ), cooled to about -20 . The mixture was added dropwise isobutylchloroformate (1.51g), then triethylamine (2.17g), stirred at the same temperature for 15 minutes. Subsequently, the reaction mixture Is added (3aR, 7aS) -2- hydroxy-hexahydro -1H- isoindole -1,3 (2H) - dione (Reference Example 22,1.86g), stirred at the same temperature for 30 minutes , it was further stirred at room temperature for 30 minutes. The reaction mixture was diluted with ethyl acetate (200 mL), with ice-cold 10% citric acid (60mL), saturated aqueous sodium bicarbonate (60mL), saturated brine (60mL) successively, dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated under reduced pressure and the residue was subjected to silica gel column chromatography (hexane / ethyl acetate = 2/1) to give the title compound as a colorless foam solid 4.521g (yield 90%). |
| 90% |
Stage #1: (2S,5R)-1-[(1,1-dimethylethyl)carbonyl]-5-[(benzyloxy)amino]piperidine-2-carboxylic acid With triethylamine; isobutyl chloroformate In tetrahydrofuran at -20℃; for 0.25h;
Stage #2: (3aR,7aS)-2-hydroxyhexahydro-1H-isoindol-1,3(2H)-dione In tetrahydrofuran at -20 - 20℃; for 1h; |
20.1 1-tert-Butyl 2-((3aR,7aS)-1,3-dioxohexahydro-1H-isoindol-2(3H)-yl)(2S,5R)-5-((benzyloxy)amino)piperidine-1,2-dicarboxylate
2S,5R)-5-((Benzyloxy)amino)-1-(tert-butoxycarbonyl)piperidine-2-carboxylic acid (Reference Example 3, Step 1, 3.504 g, 10 mmol) was dissolved in dehydrated tetrahydrofuran (50 mL), followed by cooling to about -20° C. To the mixture were added dropwise isobutyl chloroformate (1.51 g) and then triethylamine (2.17 g), followed by stirring at the same temperature for 15 minutes. Then, to the reaction solution was added (3aR,7aS)-2-hydroxyhexahydro-1H-isoindol-1,3(2H)-dione (Reference Example 19, 1.86 g), followed by stirring at the same temperature for 30 minutes and then at room temperature for 30 minutes. The reaction solution was diluted with ethyl acetate (200 mL), washed sequentially with ice-cold 10% citric acid (60 mL), saturated sodium bicarbonate (60 mL), and saturated brine (60 mL), dried over anhydrous magnesium sulfate, and filtered. The residue obtained by concentrating the filtrate under reduced pressure was subjected to silica gel column chromatography (hexane/ethyl acetate=2/1) to afford 4.521 g of the title compound as a colorless foamy solid (yield 90%). 1H NMR (400 MHz, CDCl3) δ 1.35-1.58 (m, 13H), 1.62 (bs, 1H), 1.76 (bs, 2H), 1.90 (bs, 4H), 1.95-2.15 (m, 2H), 3.00 (bs, 2H), 3.15-3.30 (m, 2H), 4.16-4.25 (m, 1H), 4.72 (q, J=11.6 Hz, 2H), 5.30-5.53 (m, 1H), 7.26-7.38 (m, 5H); MS m/z 502 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping