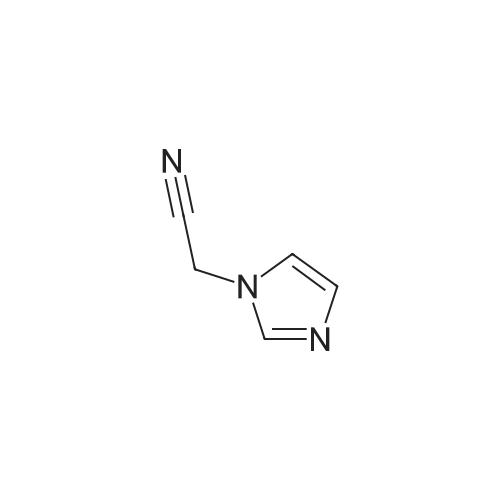
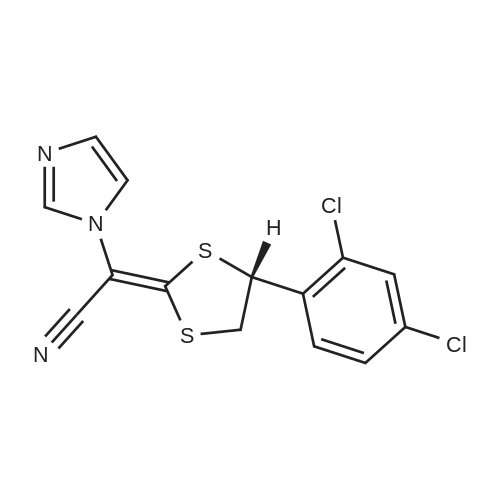
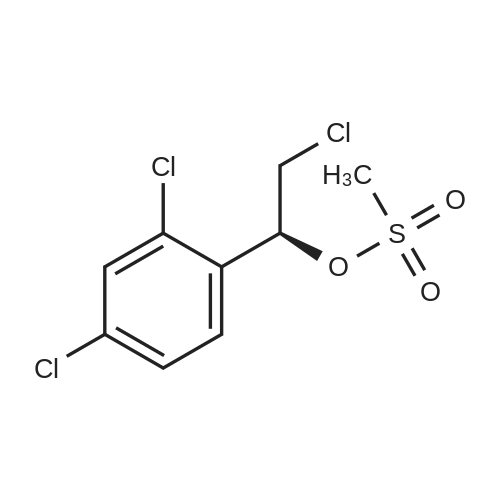
| 51.97% |
In acetonitrile at -5 - 0℃; for 20h; Large scale; |
1.1.2-1.1.3; 1.2.i-1.2.ii; 1.3.a-1.3.d; 2-18
After the reaction in the previous step, reduce the temperature in the kettle to -5-0°C, and slowly add (S)-2-chloro-1-(2,4-dichlorophenyl)ethyl methanesulfonate within this temperature range The acetonitrile solution of methanesulfonate (compound II) (from 58.9kg (S)-2-chloro-1-(2,4-dichlorophenyl)ethyl methanesulfonate (approximately 194 mol, CAS number: 229334) -55-8, molecular formula: C9H9Cl3O3S) Prepared by dissolving in 235.2kg of acetonitrile), the feeding rate is 28kg/h35kg/h, after the addition, continue to stir and react for 20h to obtain a reaction solution containing compound I (luliconazole);. Slowly add an appropriate amount of purified water (about 560kg in this example) to the reaction solution of the previous step, control the temperature of the system at -5°C to 0°C, stir, precipitate solids, filter, and dry (control moisture ≤2.0%) , 67.35 kg of crude luliconazole was obtained, which was a pale yellow or off-white solid, with a purity of 68.8% and an ee value of 97.50%. in,Purity refers to the mass percentage content of the target product in all output (including: target product, unreacted raw materials and other impurities).ee value, optical purity, refers to enantiomeric excess (enantiomeric excess), which means the excess of one enantiomer to another, usually expressed as a percentage. For definition and explanation, please refer to Chapter 3 of "Design of Drug Synthesis" Page 153 (Editor-in-Chief Zhang Wannian, Second Military Medical University Press, 2010 Edition).2. Production of Luliconazole HydrochlorideThe production method of luliconazole hydrochloride includes the following steps:i. Under the protection of inert gas (for example, nitrogen, etc.), add 67.35kg of crude luliconazole (obtained in the previous step, purity of 68.8%, equivalent to about 130.7mol containing luliconazole), 774.5kg of tetrahydrofuran and 40.5kg into the reactor Ethyl acetate, turn on the stirring, the stirring rate is 100r/min550r/min (250r/min is used in this embodiment), and the temperature in the kettle is controlled to 520 (considering energy saving and consumption reduction, this embodiment is preferably 10 20), pass 10.4kg (about 285mol) of hydrogen chloride gas into the above system, and the feed rate is 0.5kg/h5kg/h (this embodiment is preferably 1.0kg/h1.5kg/ h) After the addition, the temperature is increased, and the reaction is refluxed for 1h-2h to obtain a reaction solution containing luliconazole hydrochloride;ii. Slowly lower the temperature of the reaction solution obtained in the previous step to 25°C30°C, precipitate a solid, filter, and dry to obtain 49.20kg of luliconazole hydrochloride (E configuration), which is a pale yellow or off-white solid with a purity of 95.0%, the ee value is 96.3%.3. Production of luliconazole refined productsThe production method of luliconazole products includes the following steps:a. Under the protection of inert gas (for example, nitrogen, etc.), add 246kg purified water, 49.2kg luliconazole hydrochloride (approximately 126mol, obtained in the previous process), 295.2kg ethyl acetate, and 26.6kg carbonic acid into the reactor. Sodium (approximately 251mol), turn on the stirring, the stirring rate is 100r/min550r/min (250r/min is used in this example), and the stirring time is 20min60min (30min in this example), and then the temperature is slowly raised to 35°C 40, continue to stir and react for 20min60min (30min in this example) to obtain a reaction solution containing luliconazole;b. Let the reaction solution obtained in the previous step stand for 15 minutes, and then perform liquid separation, take the organic phase, extract the aqueous phase with ethyl acetate (148L×2), combine the organic phases, add anhydrous sodium sulfate to dry, filter, Add 7.5 kg of powdered activated carbon to the filtrate, then heat to reflux and stir for 1 hour, then cool to 25°C to 30°C, and filter to obtain a yellow to colorless transparent liquid;c. Perform vacuum distillation on the liquid obtained in step b above, and distill until there is no fraction to obtain a light yellow solid;d. Add 178kg methanol to the light yellow solid obtained in the above step c, heat to reflux, stir for 20min-60min (for example, 30min), slowly add purified water (approximately 89kg) under reflux, and then slowly lower the temperature to 2530, and precipitate The solid was filtered and dried to obtain 35.72 kg of luliconazole product, which was a white crystalline solid with a purity of 99.0% and an ee value of 99.5%;Based on (S)-2-chloro-1-(2,4-dichlorophenyl)ethyl methanesulfonate, the yield of luliconazole product was 51.97%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping