|
With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In water; N,N-dimethyl-formamide; for 14h; |
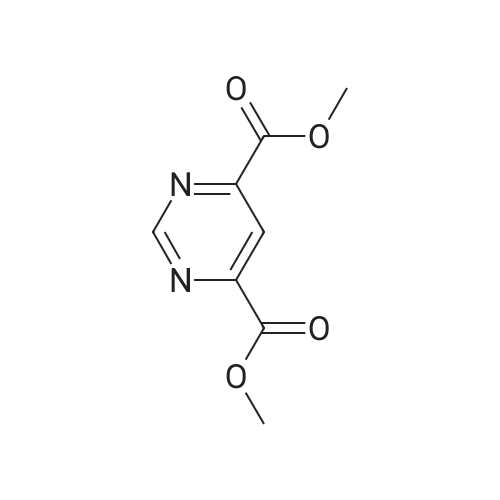
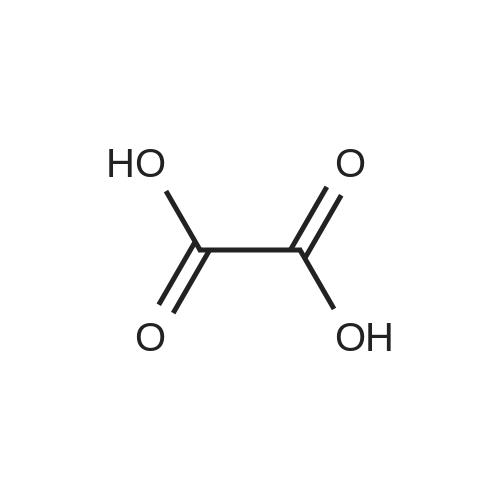
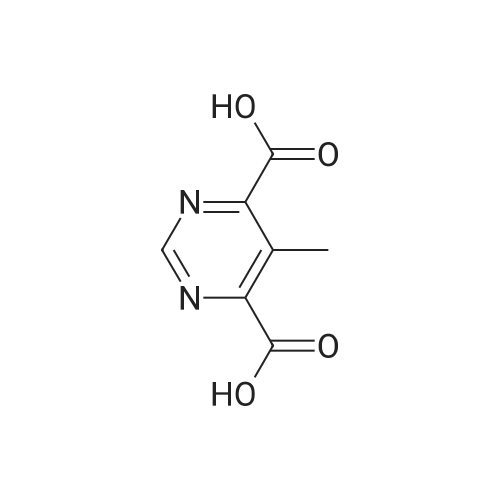
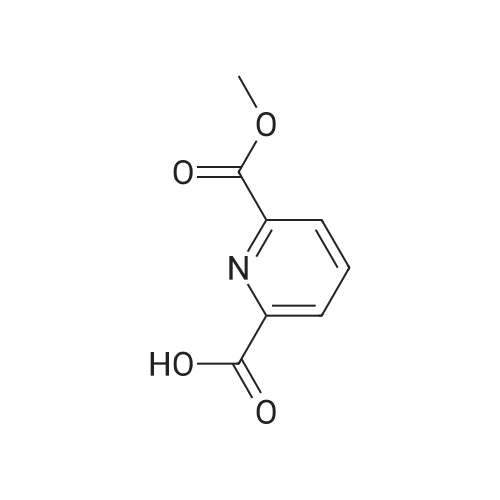
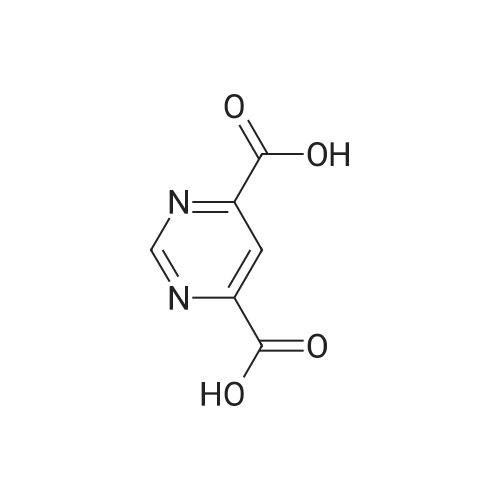
Next, to a suspension of pyrimidine«4,6-dicarboxytic acid (325 mg, 1.935 mmol), (R)-Z- amino-4-biphenyl-4-yl-butyric acid ethyl ester hydrochloride (250 mg, 0.774 mmol), WSC hydrochloride (148 mg, 0.774 mmol) and HOAt (105 mg, 0.774 mmol) in DMF (4 mL) and H2O (1 mL) is added DIPEA (0.135 mL, 0.774 mmol). After stirring for 14 hours, the reaction is quenched with H2O, and the products are extracted with EtOAc, washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The obtained residue is purified by RP-HPLC (SunFire C18, H2O(0.1% TFA)/CH3CN), and then lyophilized to give (R)-6-(1-(biphenyl-4-yl)-4-ethoxy-4-oxobutan-2- ytearbamoyl)py?midine-4-carboxyi»c acid (84.8 mg). HPLC retention time = 1.32 minutes (condition B); MS (m+1) = 434.1 ; 1H NMR (400 MHz, DMSO-t/6) delta ppm 1.12 (t, J = 7.0 Hz1 3 H) 2.65 (A of ABX, Jab « 15.4 Hz, Jax = 5.8 Hz1 1 H) 2.73 (B of ABX, Jab ~ 15.4 Hz, Jbx * 7.9 Hz) 2.91 (A of ABX1 Jab - 13.6 Hz, Jax « 6.1 Hz, 1 H) 3.01 (B of ABX1 Jab ~ 13.6 Hz, Jbx s 8.2 Hz1 1 H) 4.01 (q, J - 7.0 Hz, 2 H) 4.59 - 4.68 (m, 1 H) 7.29 - 7.35 (m, 3 H) 7.41 - 7.45 (m, 2 H) 7.55 ~ 7.63 (m, 4 H) 8.32 (d, J = 1.35 Hz1 1 H) 9.19 (d. J = 9.1 Hz, 1 H) 9.50 (d, J = 1.35 Hz, 1 H) 14.11 (br s, 1 H). |
| 84.8 mg |
With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In water; N,N-dimethyl-formamide; for 14h; |
To (R)-ethyl-4-(biphenyl-4-yl)-3-(tert-butoxycarbonylamino)butanoate (300 mg, 0.782 mmol) is added a solution of 4M HCl in 1,4-dioxane (3.92 mL, 15.65 mmol) at room temperature. After stirring for 1 hour, the reaction mixture is concentrated under reduced pressure to give (R)-3-amino-4-biphenyl-4-yl-butyric acid ethyl ester hydrochloride. (0265) Next, to a suspension of <strong>[16490-02-1]pyrimidine-4,6-dicarboxylic acid</strong> (325 mg, 1.935 mmol), (R)-3-amino-4-biphenyl-4-yl-butyric acid ethyl ester hydrochloride (250 mg, 0.774 mmol), WSC hydrochloride (148 mg, 0.774 mmol) and HOAt (105 mg, 0.774 mmol) in DMF (4 mL) and H2O (1 mL) is added DIPEA (0.135 mL, 0.774 mmol). After stirring for 14 hours, the reaction is quenched with H2O, and the products are extracted with EtOAc, washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. (0266) The obtained residue is purified by RP-HPLC (SunFire C18, H2O(0.1% TFA)/CH3CN), and then lyophilized to give (R)-6-(1-(biphenyl-4-yl)-4-ethoxy-4-oxobutan-2-ylcarbamoyl)pyrimidine-4-carboxylic acid (84.8 mg). HPLC retention time = 1.32 minutes (condition B); MS (m+1) = 434.1; 1H NMR (400 MHz, DMSO-d6) delta ppm 1.12 (t, J = 7.0 Hz, 3 H) 2.65 (A of ABX, Jab = 15.4 Hz, Jax = 5.8 Hz, 1 H) 2.73 (B of ABX, Jab = 15.4 Hz, Jbx = 7.9 Hz) 2.91 (A of ABX, Jab = 13.6 Hz, Jax = 6.1 Hz, 1H) 3.01 (B of ABX, Jab = 13.6 Hz, Jbx = 8.2 Hz, 1 H) 4.01 (q, J = 7.0 Hz, 2 H) 4.59 - 4.68 (m, 1 H) 7.29 - 7.35 (m, 3 H) 7.41 - 7.45 (m, 2 H) 7.55 - 7.63 (m, 4 H) 8.32 (d, J = 1.35 Hz, 1 H) 9.19 (d. J = 9.1 Hz, 1 H) 9.50 (d, J = 1.35 Hz, 1 H) 14.11 (brs, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping