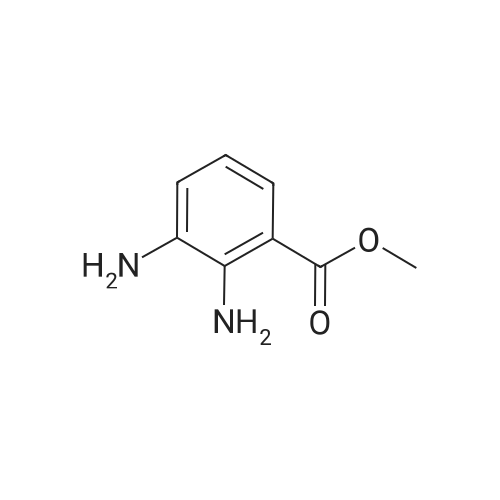
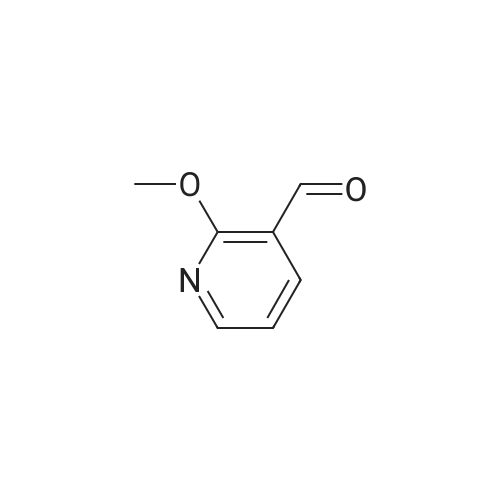
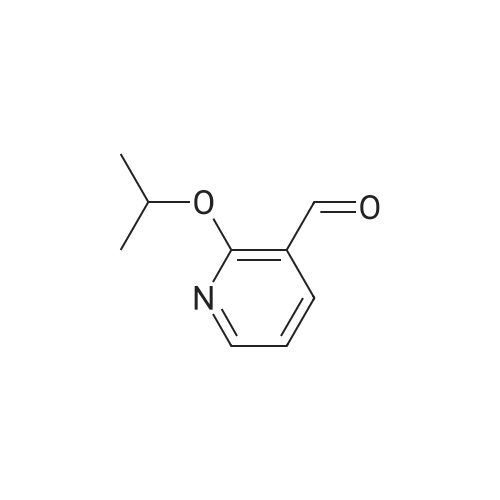
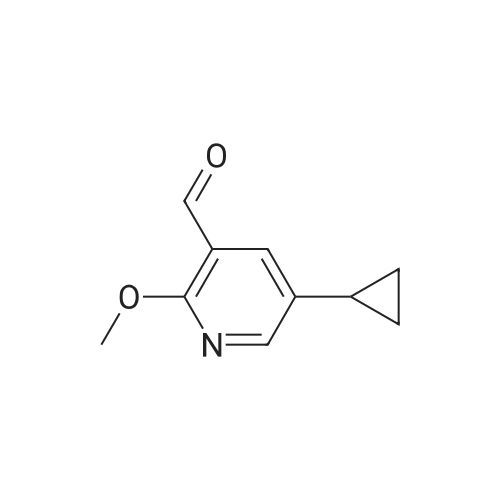
|
With triethylsilane; trifluoroacetic acid; |
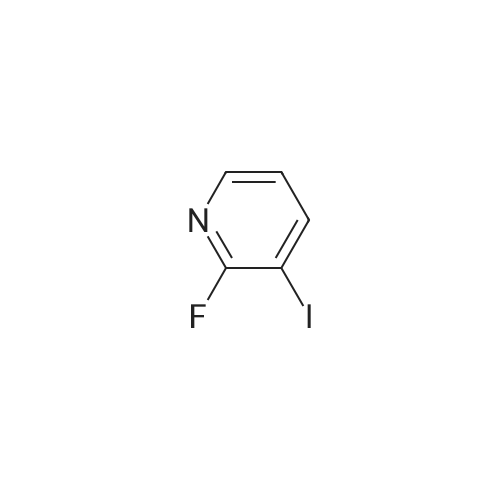
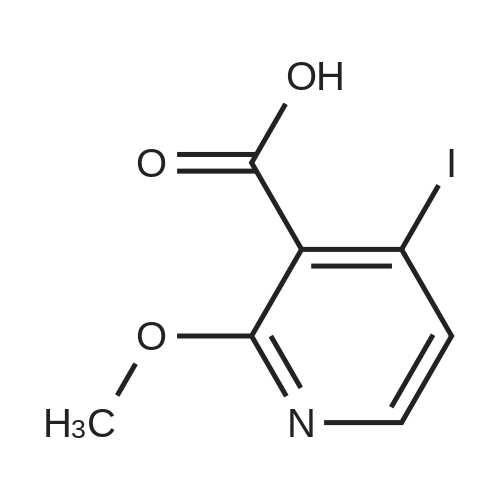
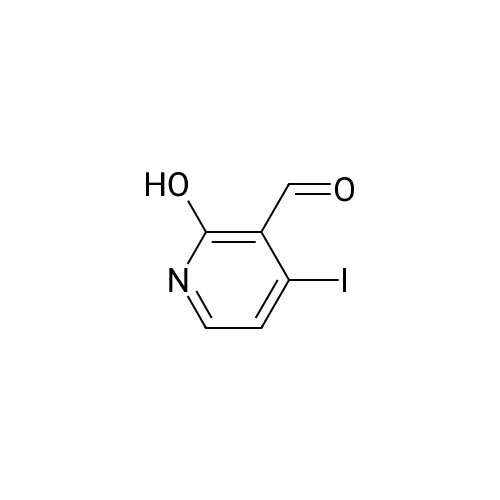
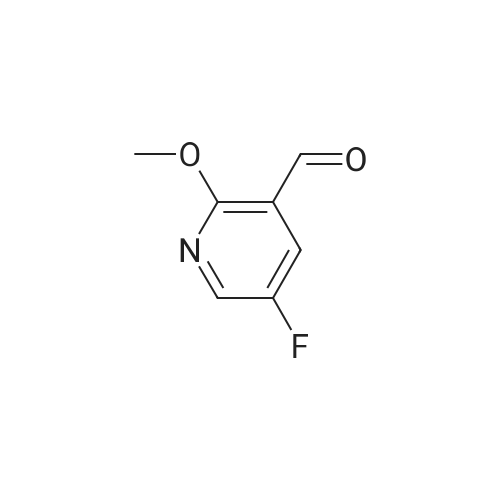
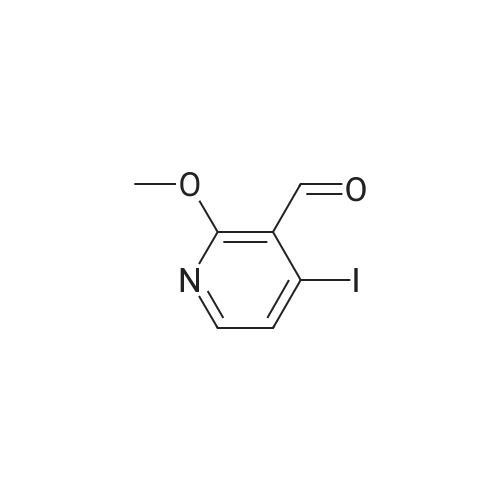
3-(But-2-enyloxymethyl)-4-iodo-2-methoxy-pyridine (Formula (XII)) A 500 mL 3-necked round-bottom flask is equipped with an overhead mechanical stirrer under nitrogen, the flask is charged with 4-iodo-2-methoxy-pyridine-3-carbaldehyde (Intermediate 1, 75.0 g, 0.29 mol), crotyl alcohol (75 mL, 0.88 mol), and triethylsilane (70 mL, 0.44 mol). To the stirred suspension at 0 C. is added trifluoroacetic acid (175 mL, 2.27 mol) dropwise via an addition funnel. The resulting solution is stirred at about 22 C. for approximately 12 hours. The reaction mixture is slowly poured into a rapidly stirring saturated sodium bicarbonate solution (2 L). The mixture is extracted with 3*500 mL of hexane. The combined organic layers are dried over sodium sulfate, filtered, and concentrated in vacuo to give an oil. Purification of this oil by vacuum distillation (about 0.4 mm Hg, about 120-130 C.) yields 3-(but-2-enyloxymethyl)-4-iodo-2-methoxypyridine as a pale yellow oil: 1 H-NMR (400 MHz, CDCl3) d 7.69 (d, J=5 Hz, 1H), 7.34 (d, J=5 Hz, 1H), 5.71 (m, 2H), 4.58 (s, 2H), 4.02 (d, J=1 Hz, 2H), 3.94 (s, 3H), 1.72 (d, J=6 Hz, 3H); IR (neat) 2948, 2859, 1561, 1459, 1381, 1361, 1301, 1233, 1169, 1094, 1052 cm-1; Elemental analysis: calculated for C11 H14 NO2 I: C 41.40, H 4.42, N 4.39, I 39.76; Found: C 41.31, H 4.45, N 4.37, I 39.71. |
|
With triethylsilane; trifluoroacetic acid; |
EXAMPLE 2 3-(but-2-enyloxymethyl)-4-iodo-2-methoxy-pyridine A 500 mL 3-necked round-bottom flask equipped with an overhead mechanical stirrer and under nitrogen, is charged with 4-iodo-2-methoxy-pyridine-3-carbaldehyde (75.0 g, 0.29 mol) as prepared in example 1, crotyl alcohol (75 mL, 0.88 mol), and triethylsilane (70 mL, 0.44 mol). To the stirred suspension at 0 C. is added trifluoroacetic acid (175 mL, 2.27 mol) dropwise via an addition funnel. The resulting solution is stirred at about 22 C. for approximately 12 hr. The reaction mixture is slowly poured into a rapidly stirring saturated sodium bicarbonate solution (2 L). The mixture is extracted with 3*500 mL of hexane. The combined organic layers are dried over sodium sulfate, filtered, and concentrated in vacuo to give an oil. Purification of this oil by vacuum distillation (ca. 0.4 mm Hg, ca. 120-130 C.) yields 3-(but-2-enyloxymethyl)-4-iodo-2-methoxy-pyridine as a pale yellow oil: 1 H-NMR (400 MHz, CDCl3) delta7.69 (d, J=5 Hz, 1H), 7.34 (d, J=5 Hz, 1H), 5.71 (m, 2H), 4.58 (s, 2H), 4.02 (d, J=1 Hz, 2H), 3.94 (s, 3H), 1.72(d, J=6 Hz, 3H); IR (neat) 2948, 2859, 1561, 1459, 1381, 1361, 1301, 1233, 1169, 1094, 1052 cm-1; Elemental analysis: calculated for C11 H14 NO2 I: C 41.40, H 4.42, N 4.39, I 39.76; Found: C 41.31, H 4.45, N 4.37, I 39.71. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping