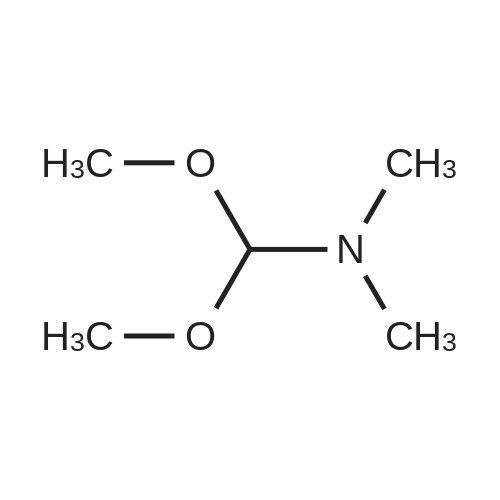
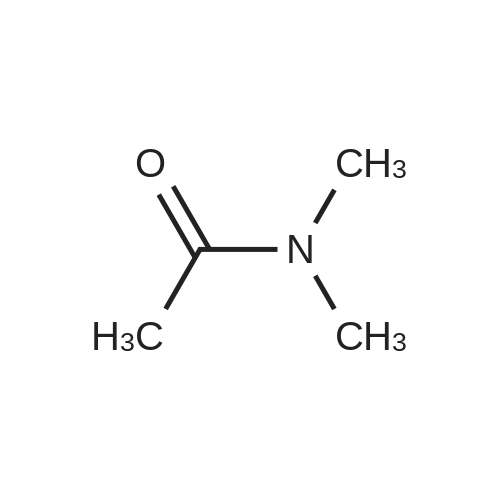
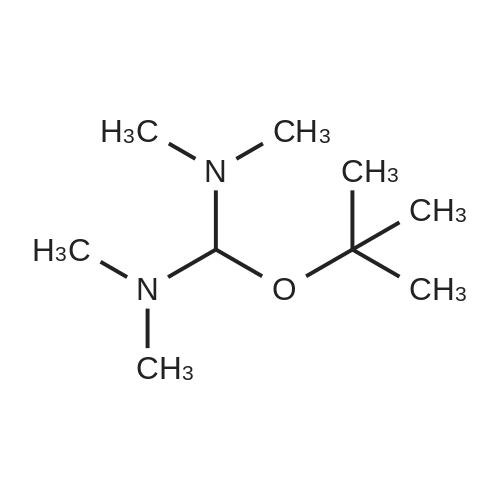
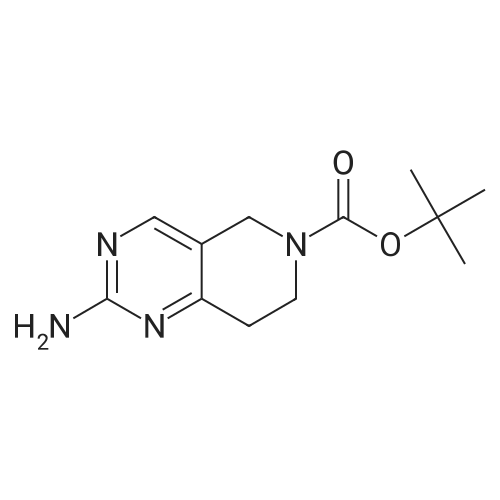
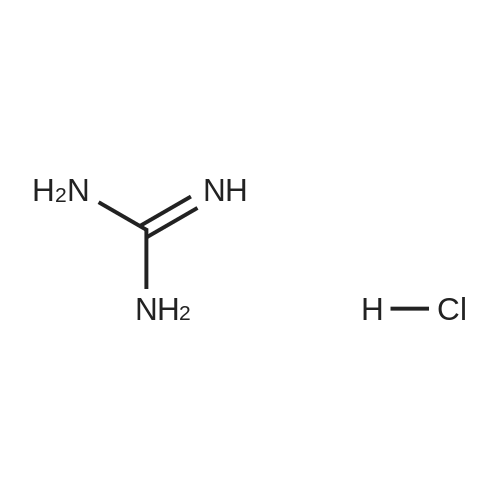
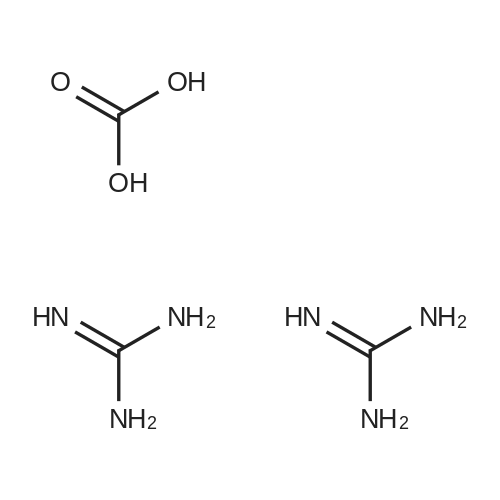
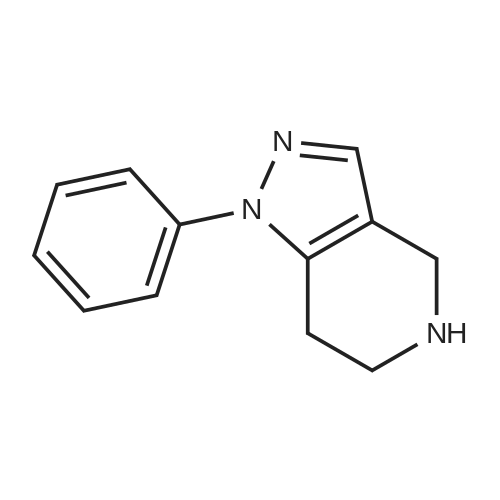
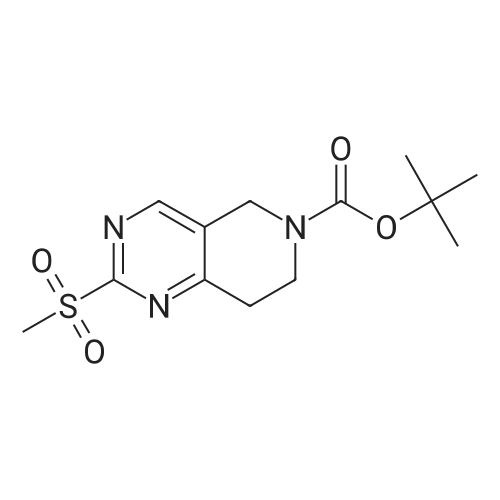
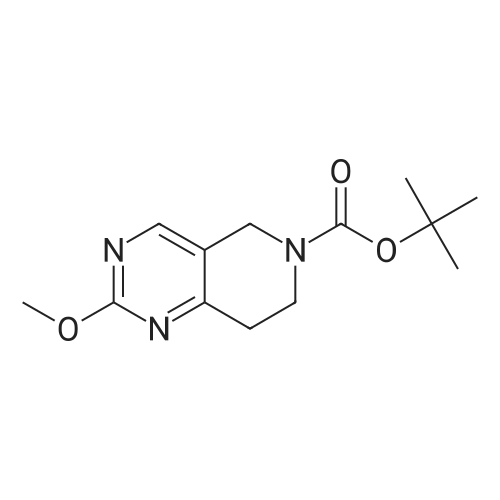
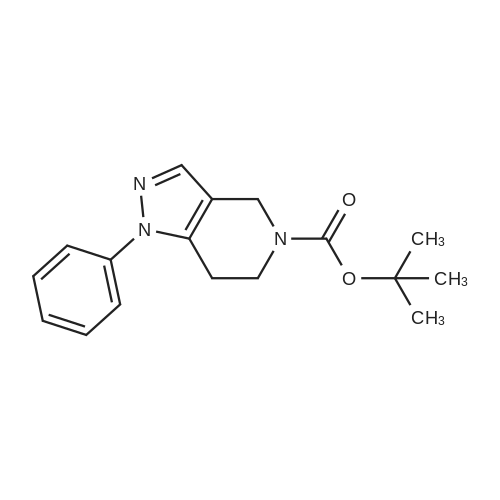
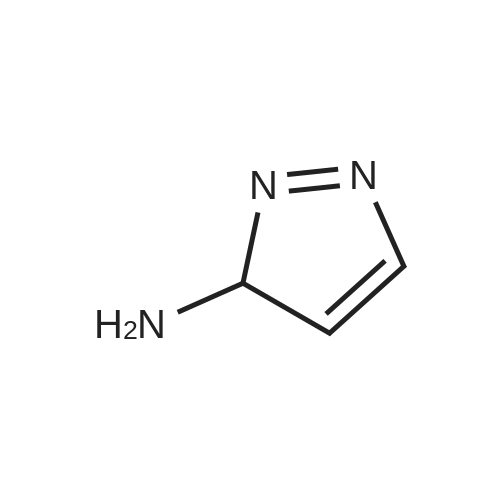
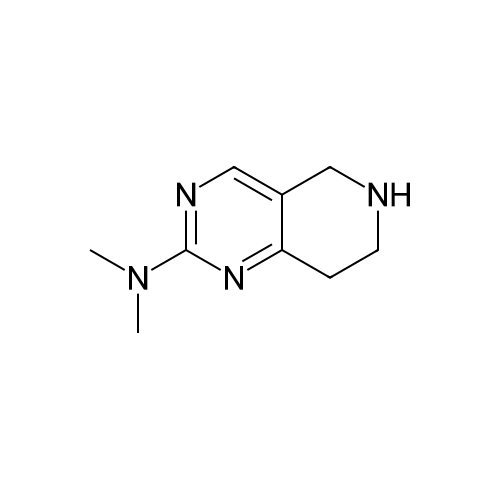
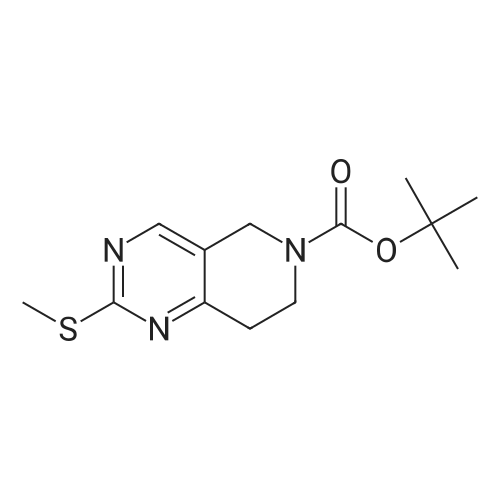
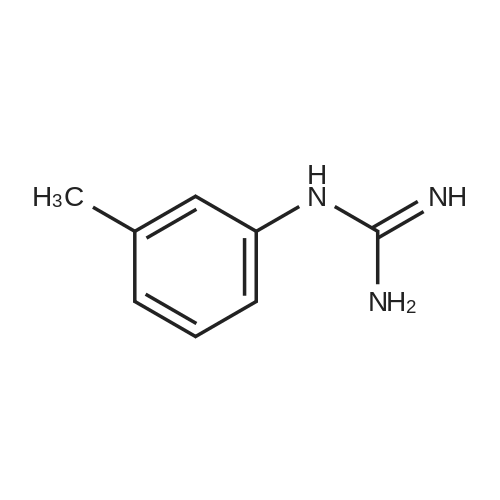
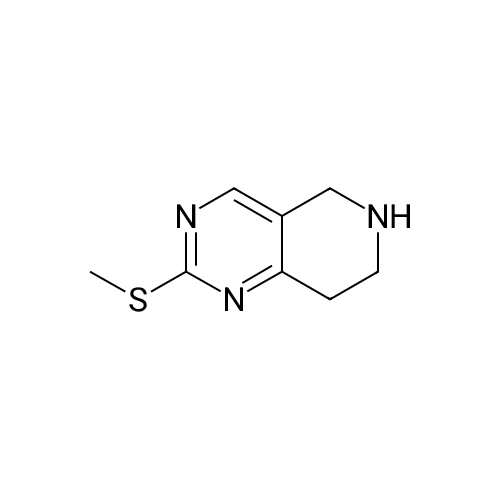
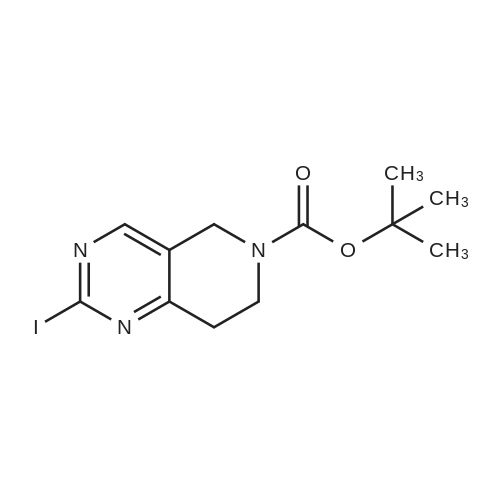
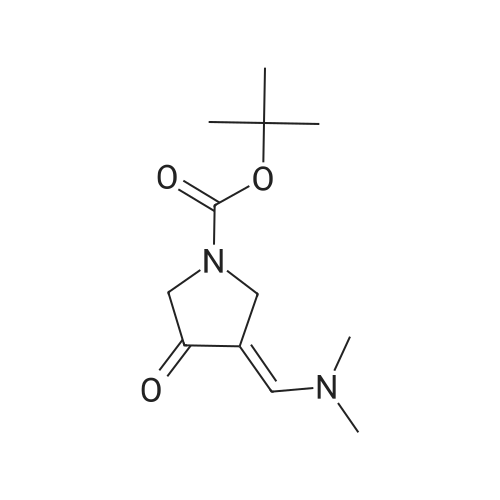
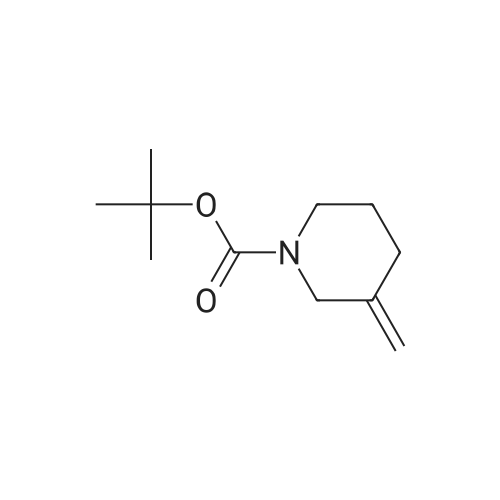
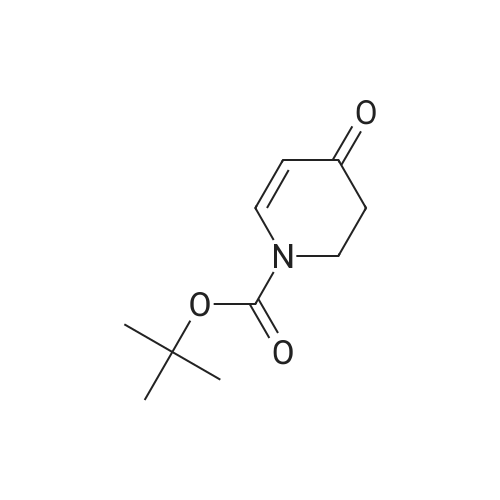
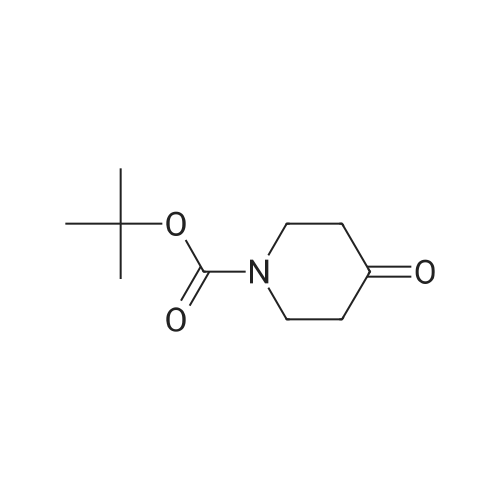
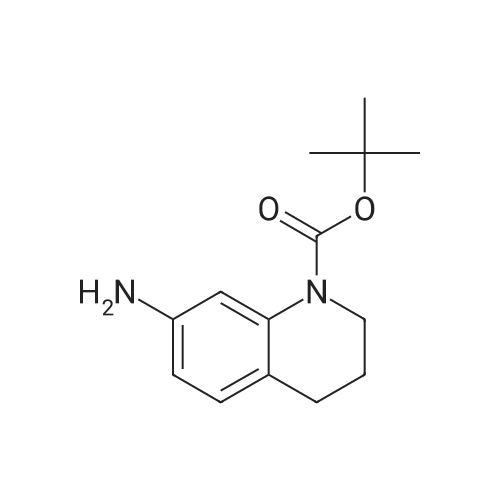
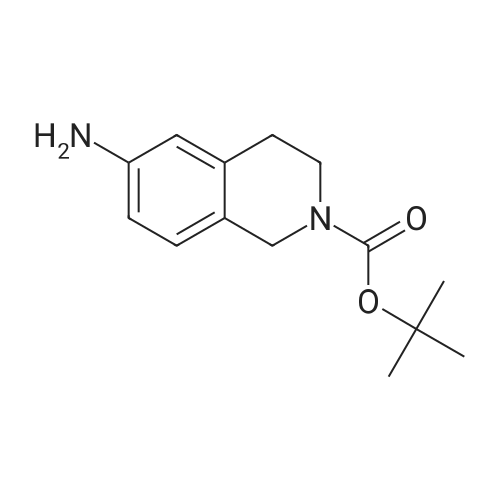
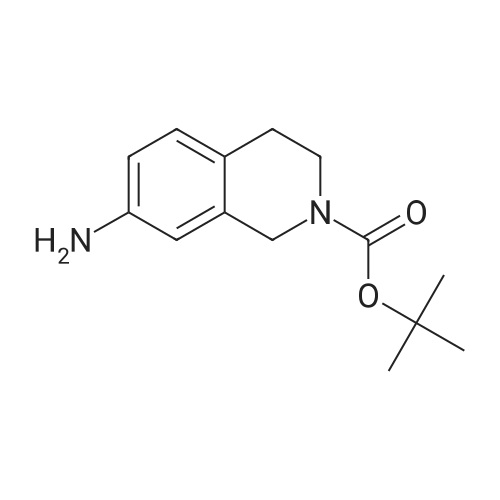
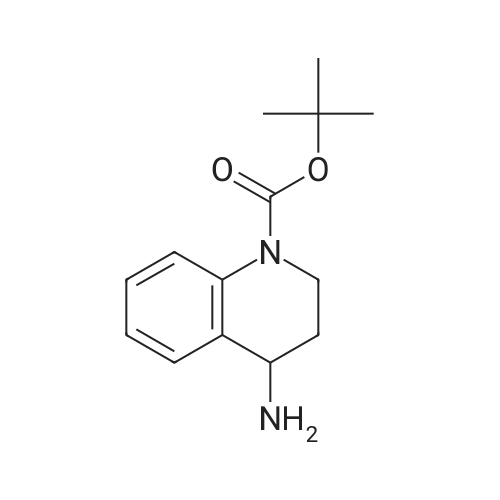
| 82% |
In 1,4-dioxane; for 20h;Reflux; |
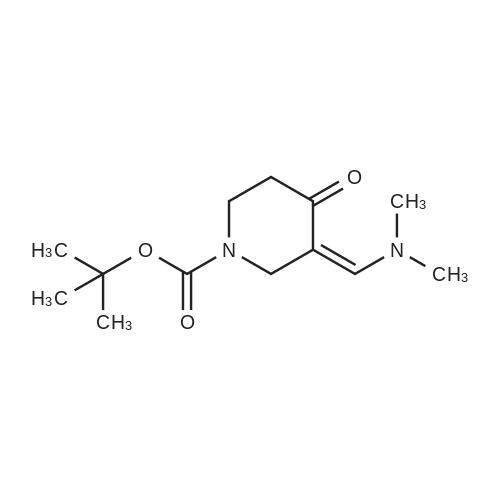
Preparation 21 t-Butyl-3-[(dimethylamino)methylidene]-4-oxopiperidine-1-carboxylate A solution of N-Boc-4-piperidone (10 g, 50.19 mmol) and DMF-dimethylacetal (20.16 ml, 150.57 mmol) in 1,4-dioxane (100 ml) was heated at reflux for 20 hours. The reaction mixture was concentrated and the residue was eluted through a flash column (silica gel 60, 230-400 mesh, 7% MeOH in EtOAc) to give the title compound as an orange oil which crystallized on standing (10.51 g, 82%). |
| 82% |
In 1,4-dioxane; for 20h;Reflux; |
Preparation 21 t-Butyl-3-[(dimethylamino)methylidene]-4-oxopiperidine-1-carboxylate A solution of N-Boc-4-piperidone (10 g, 50.19 mmol) and DMF-dimethylacetal (20.16 ml, 150.57 mmol) in 1,4-dioxane (100 ml) was heated at reflux for 20 hours. The reaction mixture was concentrated and the residue was eluted through a flash column (silica gel 60, 230-400 mesh, 7% MeOH in EtOAc) to give the title compound as an orange oil which crystallized on standing (10.51 g, 82%). |
| 76% |
In N,N-dimethyl-formamide; |
(a) 1 -(t-Butyloxycarbonyl)-3-(N,N-dimethylaminomethylidene)-4-piperidone DMF dimethyl acetal (5.82 ml, 0.044 mol) was added to a stirred solution of 1-boc-4-piperidone [Ashwood et al., J. Chem. Soc., Perkin 1, 641 (1995)] (8.73 g, 0.044 mol) in DMF (80 ml) and the reaction mixture was heated to 80 C. under N2 for 18 h. After cooling, the DMF was removed under reduced pressure and the residue was partitioned between EtOAc and H2O, the organic layer washed with H2O and saturated brine, then dried over MgSO4 and evaporated to afford the subtitle compound as a solid (8.44 g, 76%). Rf 0.33 (CH2Cl2/MeOH/0.88NH3 90/10/1, v/v). MS m/z 255 (MH+). |
| 76% |
In N,N-dimethyl-formamide; at 80℃; for 18h;Inert atmosphere; |
Step 1DMF dimethyl acetal (5.82 mL, 0.044 mol) was added to a stirred solution of tert-butyl 4-oxopiperidine-l-carboxylate (8.73 g, 0.044 mol) in DMF (80 mL) and the reaction mixture was heated to 80 C under N2 for 18 h. After cooling, the DMF was removed under reduced pressure and the residue was partitioned between EtOAc and H20. The organic layer was washed with H20 and saturated brine, then dried over MgS04 and evaporated to afford tert- butyl 3-((dimethylamino)methylene)-4-oxopiperidine-l-carboxylate (8.44 g, 76%). |
| 63.8% |
for 1.5h;Reflux; |
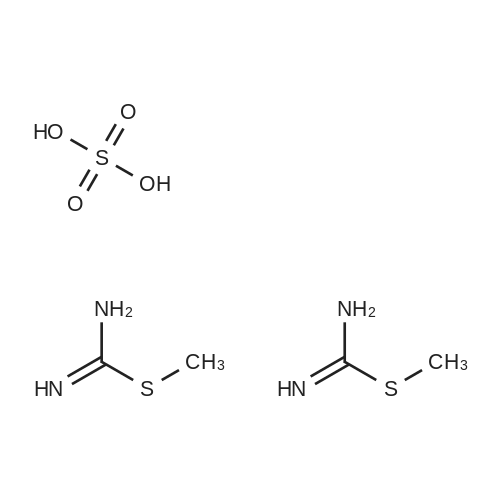
N-tert-butoxycarbonyl-4-piperidone (58, 5.8 g, 31.4 mmol) wasdissolved in N,N-dimethylformamide dimethyl acetal (45.0 mL),and the solution was heated under reflux for 1.5 h and concentrated.The residue was triturated with hexane, filtered, andwashed with hexane to give 59 as a yellow powder (5.1 g, 63.8%):mp 135e136 C; To a solution of 59 (5.0 g, 20.8 mmol) in EtOH(200.0 mL) were added guanidine carbonate (15.0 g, 84.0 mmol)and sodium acetate (13.7 g, 167.0 mmol), and the solution washeated under reflux for 48 h. The reaction mixturewas filtered, andthe insoluble material was extracted with CHCl3 and washed withwater. The organic layer was dried over anhydrous MgSO4 andevaporated. The resultant solid was triturated with 2-propanol,filtered, and washed with 2-propanol and Et2O to give a colorlesspowder. It was dissolved in TFA (50.0 mL) at 0 C, and the solutionwas stirred at room temperature for 1 h and concentrated. Theresidue was dissolved in 2-propanol and treated with concentrated HCl (4.0 mL). The precipitated solidwas filtered andwashed with 2-propanol and Et2O to give 60a (4.2 g, 81.6%) as a colorless powder: Mp 258e260 C; Compound 57g was obtained from 60a in thesame way as 57f. |
| 60% |
In 1,4-dioxane; for 15h;Reflux; |
A solution of N-t-Butoxycarbonyl-4-piperidone (10.0 g, 50.19 mmol) and N,N-dimethylformamide dimethylacetal (20.16 mL, 150.57 mmol) in 1,4-dioxane (100 mL) was heated at reflux for 15 hours. The solvent was removed in vacuo and the residue was eluted through a flash column (silica gel 60, 230-400 mesh, 8% MeOH in EtOAc) to obtain the title compound as an orange oil which crystallized on standing (7.64 g, 60%). |
| 40% |
for 6h;Reflux; |
Stage 1: tert-Butyl 3-((dimethylamino)methylene)-4-oxopiperidine-1-carboxylate A mixture of tert-butyl 4-oxopiperidine-1-carboxylate (20.0 g, 100.38 mmol, 1.0 eq.) and N,N-dimethylformamide dimethylacetal (80 ml) was refluxed for 6 hours. After monitoring by thin-layer chromatography, the reaction mixture was concentrated under reduced pressure and the residue was taken up in DCM (300 ml), washed with water (200 ml) and sat. NaCl solution (200 ml), dried over sodium sulfate, concentrated and purified by column chromatography (silica gel, 2.5% MeOH in DCM). Yield: 40% (10.2 g, 40.16 mmol) |
|
for 6h;Heating / reflux; |
A mixture of 1-tert-butoxycarbonylpiperidine-4-one and N,N-dimethylformamide dimethylacetal was stirred for 6 hours heated to reflux to give 1-tert-butoxycarbonyl-3-[(dimethylamino)methylene]piperidine-4-one. A mixture of the obtained 1-tert-butoxycarbonyl-3-[(dimethylamino)methylene] piperidine-4-one, 2-hydrazinoethanol and MeOH was stirred for two hours heated to reflux to give a mixture of 2-(5-tert-butoxycarbonyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]pyridine-2-yl)ethanol and 2-(5-tert-butoxycarbonyl-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridine-1-yl)ethanol. A mixture of the obtained mixture, 4M HCl-EtOAc solution and EtOH was stirred for two hours at room temperature to give a mixture of 2-(4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]pyridine-2-yl)ethanol dihydrochloride and 2-(4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c] pyridine-1-yl)ethanol dihydrochloride. ES-MS(+) : 168 |
|
In N,N-dimethyl-formamide; at 90℃; for 16h; |
tert-Butyl-4-oxo-l-piperidinecarboxylate (10 g, 50 mmol) and N,N-dimethylformamide dimethyl acetal (7.3 ml, 55 mmol) are added to dry DMF (75 ml) under nitrogen, and the mixture is heated at 90 0C for 16 h. The reaction mixture is concentrated under reduced pressure, and the residue is partitioned between EA (200 ml) and brine (200 ml). Layers are separated and the aqueous phase is extracted with EA (200 ml). The combined organic extracts are dried over sodium sulfate and concentrated under reduced pressure to give a brown oil that solidifies on standing. The material is used for the next step reaction without further purification. 1H NMR (300 MHz, CDCl3) delta 7.44 (IH, s),4.54 (2H, s), 3.59 (2H, t), 3.06 (6H, s), 2.42 (2H, t), 1.44 (9H, s). |
|
In toluene; at 100℃; for 2.5h; |
tert-Butyl 4-oxo-1-piperidinecarboxylate (0.98 g, 4.9 mmol) and N,N'-dimethylformamide dimethyl acetal (0.65 ML, 4.9 mmol) were suspended in toluene and stirred at 100 C. for 2.5 h.The resulting solution was concentrated in vacuo to a constant weight. MS: m/z 256 (M+2).To this oil was added 2-(2-(4-pyridyl)-1,3-thiazol-4-yl)acetamide (Example 18b) (1.10 g, 5.0 mmol), 20 ML of anhydrous DMF, and finally NaH (60% in mineral oil, 0.34 g, 8.5 mmol) in one portion.The resulting solution was stirred at RT over the weekend.The solution was diluted with H2O and acidified to PH ~4.The resulting precipitate was filtered and washed with H2O. The solid was stirred in 150 ML of hexanes and filtered to give a tan solid. MS m/z: 411 (M+1). Calc'd for C21H22N4O3S Exact Mass: 410.14. |
|
for 6h;Heating / reflux; |
Reference Example 15 A mixture of 1-tert-butoxycarbonylpiperidin-4-one and N,N-dimethylformamide dimethylacetal was heated under reflux with stirring for 6 hours to yield 1-tert-butoxycarbonyl-3-[(dimethylamino)methylene]piperidin-4-one. A mixture of 1-tert-butoxycarbonyl-3-[(dimethylamino)methylene]piperidin-4-one obtained, 2-hydrazinoethanol, and MeOH was heated under reflux with stirring for 2 hours to yield a mixture of 2-(5-tert-butoxycarbonyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]pyridin-2-yl)ethanol and 2-(5-tert-butoxycarbonyl-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridin-1-yl)ethanol. A mixture of the compounds obtained, 4M HCl-EtOAc solution, and EtOH was stirred at ambient temperature for 2 hours to yield a mixture of 2-(4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]pyridin-2-yl)ethanol dihydrochloride and 2-(4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridin-1-yl)ethanol dihydrochloride. ME: 168 |
|
In N,N-dimethyl-formamide; at 100℃; for 60h; |
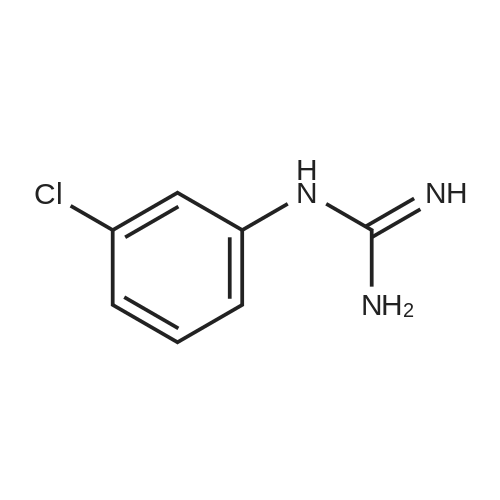
Preparation 2W-(3-Chlorophenyl)-5,6,7,8 etrahydropyrido[4,3-d]pyrimidin-2-amine[00135]fe/f-Butyl 4-oxopiperidine-1 -carboxylate (2.0 g, 10.04 mmol) is dissolved in DMF (2.5 ml_) and DMF-DMA (1 .67 g, 14.05 mmol) is added. The mixture is heated at 100 C for 60 h, then cooled and evaporated to dryness to give fe/f-butyl 3- ((dimethylamino)methylene)-4-oxopiperidine-1 -carboxylate. This intermediate (1 .0 g, 3.93 mmol) is dissolved in abs. EtOH (20 ml_), and 3-chlorophenylguanidine (0.444 g, 2.62 mmol) is added. The mixture is heated at 100 C for 16 h, then cooled and evaporated to dryness. Purification of the residue by flash column chromatography provides fe/f-butyl 2-((3-chlorophenyl)amino)-7,8-dihydropyrido[4,3-d]pyrimidine-6(5/-/)- carboxylate (0.54 g, 57%) as a yellow solid. This intermediate (0.085 g, 0.23 mmol) is dissolved in DCM (5 ml_), TFA (1 ml_) is added and the mixture is stirred at room temperature for 6 h. The mixture is evaporated and partitioned between DCM and saturated aqueous NaHC03 solution. The organic phase is separated, dried over anhydrous Na2S04 and evaporated to give the title compound (60 mg, 98%) as a brown solid. |
|
In N,N-dimethyl acetamide; at 80℃; for 12h; |
[0157] Method I-Step a: Tert-butyl3-((dimethylamino)methylene)-4-oxopiperidine-l- carboxylate [0158] A solution of tert-butyl 4-oxopiperidine-l-carboxylate (10 g, 50.25 mmol) and 1,1- dimethoxy-N,N-dimethylmethanamine (6 g, 50 mmol) in 40 mL of dry N,N- dimethylacetamide was heated at 80 C for 12 hours. The solution was cooled down and concentrated under reduced pressure, and the residue was portioned between ethyl acetate (150 mL) and water (50 mL). The organic layer was washed with brine (50 mLx2), dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford an orange oil for next step without further purification (13 g). |
|
In 1,4-dioxane; at 100℃; for 20h; |
A solution of 4-oxo-1-piperidinecarboxylic acid 1,1-dimethylethyl ester (25 g, 126 mmol) and DMF-DMA (50 mL, 377 mmol) in 1,4-dioxane (200 mL) was heated at 100 C for 20 hours. Upon completion, the reaction mixture was concentrated under reducedpressure to yield the title compound, which was used without further purification. |
|
at 120℃; for 4h;Inert atmosphere; |
Step 1: Preparation of tert-butyl 2-pyrimidin-2-yl-7,8-dihydro-5H-pyrido[4,3- d]pyrimidine-6-carboxylate A solution of N-(tert-butoxycarbonyl)-4-piperidone (100.0 g, 0.50 mol) in DMFDMA (299.0 g, 2.5 mol) was heated with stirring at 120 oC under N2 for 4 hrs. The resulting reaction mixture was concentrated in vacuo. The residue was dissolved in MeOH (2.0 L), and to the resulting solution was added 2-amidinopyrimidine hydrochloride (87.8 g, 0.55 mol) and K2CO3 (173.9 g, 1.26 mol) successively. The resulting mixture was heated with stirring at 70 oC for 3 hrs. The resulting reaction mixture was cooled to rt and filtered. The filtrate was concentrated in vacuo. The residue was diluted with DCM (2.0 L), washed with H2O (500 mL) and brine (300 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by the flash column chromatography to give tert-butyl 2-pyrimidin-2-yl-7,8-dihydro-5H-pyrido[4,3- d]pyrimidine-6-carboxylate (47.7 g) as a yellow solid. |
|
In N,N-dimethyl-formamide; at 90℃; |
A mixture of tert-butyl 4-oxopiperidine-1-carboxylate (15.0 g, 0.075 mol) and DMFDMA (9.87 g, 0.0829 mol) in DMF (100 mL) was heated at 90 C with stirring overnight. The resulting mixture was then concentrated in vacuo and diluted with water (100 mL). The resulting mixture was extracted with EA (30 mL) for three times. The combined organic layer was washed with water, dried over anhydrous Na2SO4 and concentrated in vacuo to give the crude tert-butyl 3-(dimethylaminomethylene)-4-oxo-piperidine-1-carboxylate (13 g) as yellow oil, which was used in the next step directly. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping