|
With triethylamine; In acetonitrile; at 78℃; for 5h; |
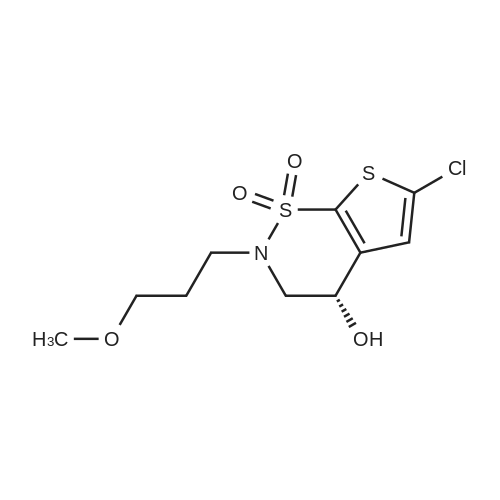
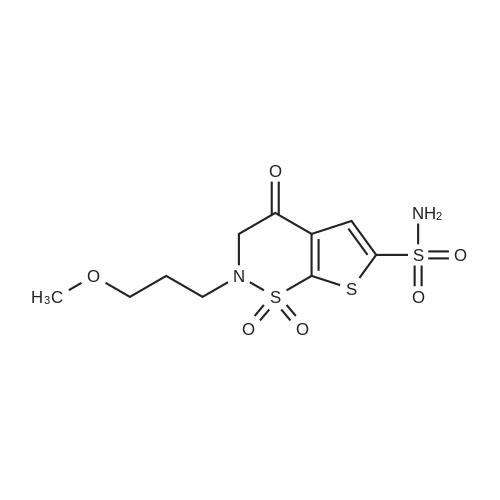
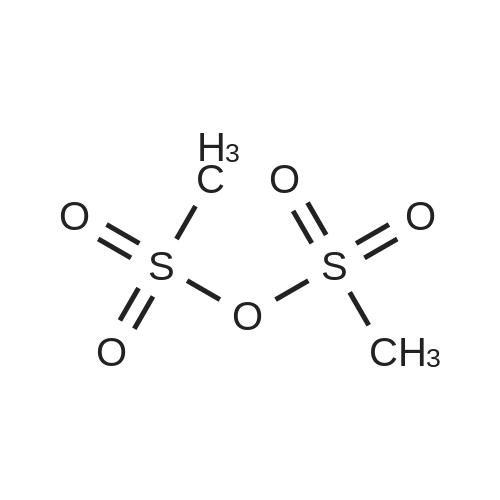
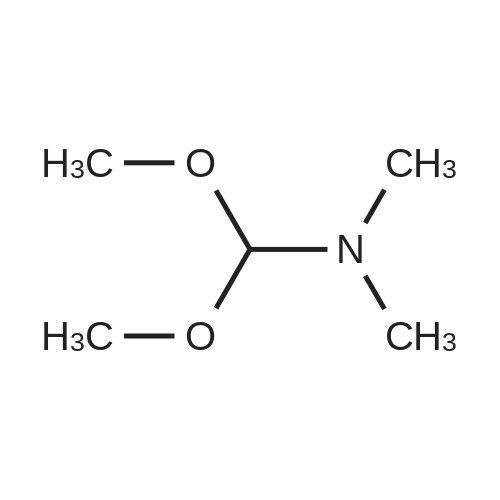
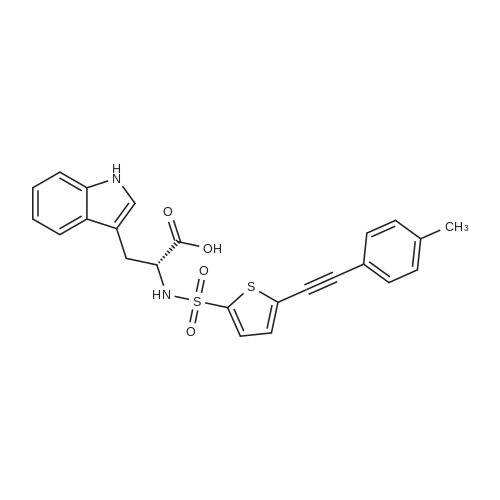
Compound B (50.0 g, 140.7 mmol) was added to 450 mL of acetonitrile, trimethyl orthoacetate (27.0 g, 225.1 mmol) and triethylamine (1.4 g, 14.0 mmol) were added with stirring, and the mixture was heated to 78C. , stirring for 5h,HPLC monitoring showed complete reaction and the HPLC monitoring results are shown in Table 1 (see FIG. 1).Cool to 40C, distill off under reduced pressure, and concentrate to a minimum volume.Compound C crude product was obtained.The crude compound C was dissolved in 180 mL of tetrahydrofuran, cooled to -10 DEG C, triethylamine (31.3 g, 309.5 mmol) was slowly added dropwise at a drip rate of 1 d/s, and the addition was completed at a drip rate of 3 d/s. 4- A solution of tosyl chloride (53.5 g, 280.6 mmol) in 70 mL of tetrahydrofuran. After the addition, the temperature was controlled at -4C, and the reaction was complete after about 3 hours.At a controlled temperature of 10 C. or less, a 70% aqueous solution of ethylamine (361.0 g, 5.6 M) was slowly added dropwise at a rate of 5 d/s. After the addition, the temperature was kept stirring at 12C and the reaction was complete after about 15 hours.The mixture was concentrated under reduced pressure to 70-80 mL, and the temperature was lowered to 0C. The temperature was controlled below 30C, and concentrated hydrochloric acid (12 mol/L) was added dropwise to adjust the pH to 1 to 2, and then about 14 mL of concentrated hydrochloric acid (12 mol/L) was added. The mixture was stirred at room temperature for 1 hour. The reaction was extracted twice with methyl tert-butyl ether (2*250 mL). The organic phases were combined and extracted once with dilute hydrochloric acid (1 mol/L, 100 mL). Combine the aqueous phases, slowly add sodium bicarbonate solids, adjust the pH to 5-6, add 150 mL of water, and adjust the pH to 7-8 with 7% sodium bicarbonate solution. After adjustment, stir at room temperature for 15 h and slowly crystallize. After filtration, the filter cake was rinsed with 30 mL of water and the cake was dried to obtain 35.4 g of product with a purity of 98.3%.Add 250 mL of dichloromethane, 25 mL of methanol to the filter mother liquor, stir, extract, separate, and concentrate the organic phase to dryness4.6 g of yellow viscous material was added and 10 mL of ethyl acetate was added. Heat to 70 ~ 75 C dissolved, slowly dropped to 0 ~ 10 C, stirring 1 ~ 2h, precipitation of a white solid, continue stirring 3 ~ 4h, filtration, with 3mL water filter cake, filter cake drying,Obtained product 2.4g, purity 97.3%.Combined, the purity was 98.1%, the total yield was 65.3%, the ignition residue was 0.08%, and the chiral purity was 99.7%. |
|
In acetonitrile; at 5 - 15℃; for 1h;Inert atmosphere; |
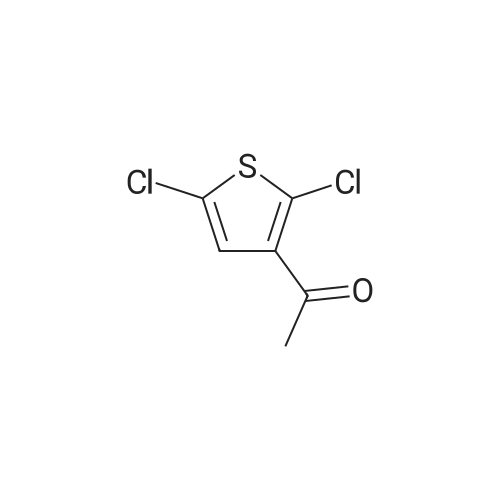
Prepare a 1L three-vial bottle equipped with a mechanical stirrer, a constant pressure low liquid funnel, and an ice water bath.Add compound of formula A (100 g, 0.28 mol) and acetonitrile (400 mL),Under N2 protection, stirring and cooling to 5 ~ 10 C,Then drop itTrimethyl orthoacetate(77.3g, 0.64mol),The rate of addition is such that the stability of the system does not exceed 15C.After the addition, remove the cooling and continue the reaction for 1 hour.TLC detected the disappearance of the starting material, creating a slightly more polar new point.Concentrate at normal pressure, recover the solvent and add the residue to DCM.Swirling to replace desolvation, and finally high vacuum drying for 1 hour,The residue is a compound of formula B-1Can be directly used in subsequent reactions without purificationThe reaction gave the crude product B-1 in equivalent yield. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping