| 73.4% |
|
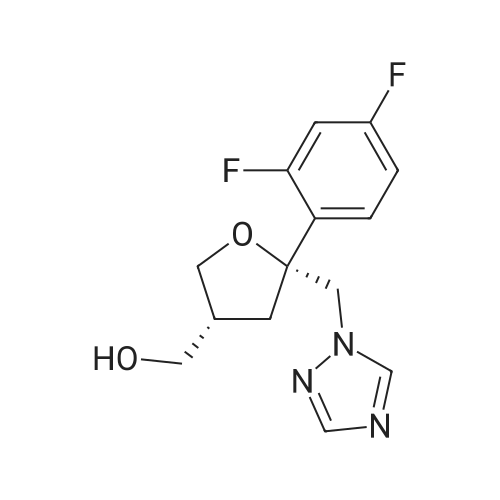
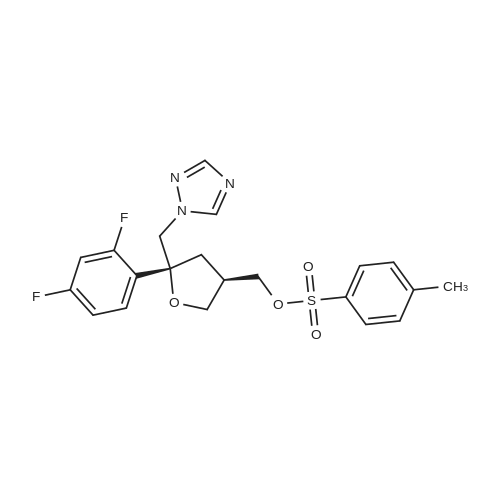
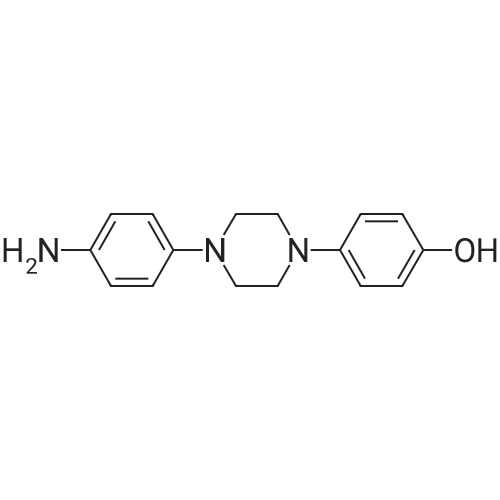
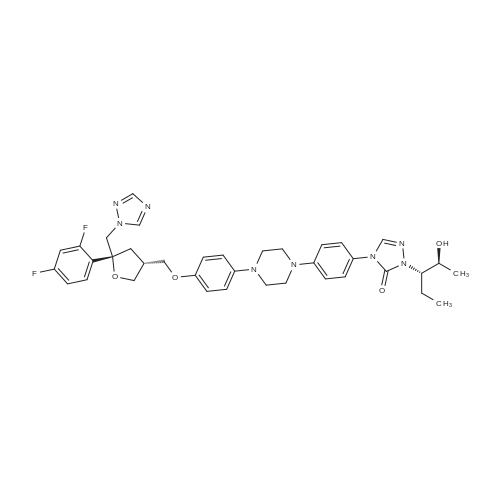
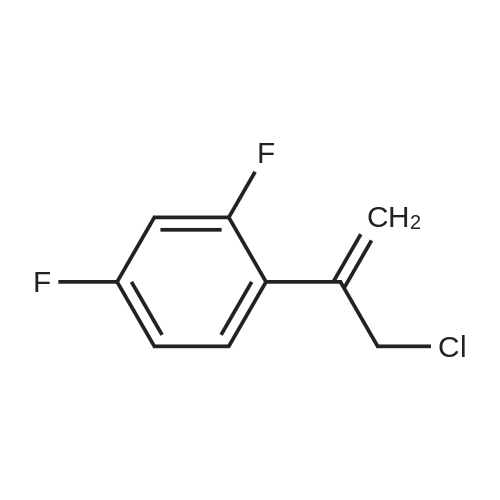
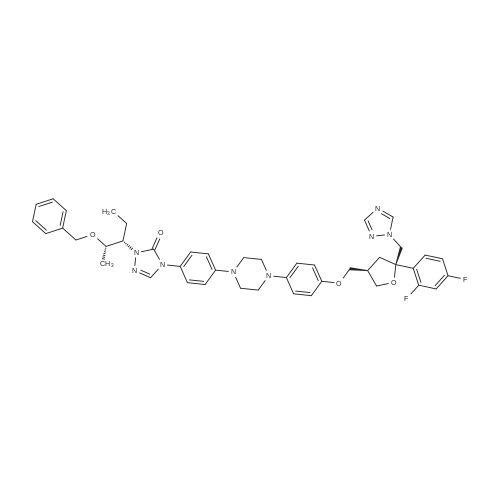
The 35.01 kg dimethyl sulfoxide, the mass concentration is 50% aqueous sodium hydroxide solution (sodium hydroxide 1.24 kg dissolved in pure water 1.24 kg, the mass concentration of the refer to sodium hydroxide of the quality of the sodium hydroxide solution the percentage of the total mass of the), adding 50L glass in the reactor, stirring solution cleaning, then adding 5.31 kg compound (III), stirring 20 - 30 minutes, adding 4.60 kg (3S, 5R) - toluene -4 - sulfonic acid 5 - (2, 4 - difluorophenyl) -5 - (1H - 1, 2, 4 - triazole -1 - yl) methyl tetrahydrofuran -3 - yl methyl ester (IV). After the completion of the feeding, temperature control 25 ± 5 C reaction 8 - 12 hours. TLC monitoring reaction, after the reaction. Control the temperature 20 - 30 C, the reaction with the sampler to 53.05 kg purification of water 100L glass reactor (rotational speed 200 - 300 rpm, dropping time 0.5 - 1.0 hours), continuously stirred for 0.5 - 1 hour. Centrifugal then adding to the basic solvent-free outflow; cake purified water 10.61 kg rear plate the basic solvent-free centrifugal to flow out. The centrifugal solid all input 50L glass in the reactor, adding 23.79 kg ethyl acetate, stirring until the solid completely dissolved. Layered, collecting the upper organic phase. Add silica gel 1.06 kg, heating to 50 - 60 C stirring 0.5 - 1 hour, cooled to 15 - 25 C, filtering. The filtrate batch transfer to 20L in the rotary evaporating bottle, at the vacuum degree of - 0.08 - - 0.1 mpa lower, control temperature 50 ± 10 C, concentrated to remove the ethyl acetate, to the solvent-free steam. To obtain brown oily matter. The oil of transfer to the 50L glass in the reactor, adding ethyl acetate 23.79 kg, heating to 50 ± 5 C, adds by drops positively hexane 36.71 kg (the dropping time 0.5 - 1.0 hours). Lowering the temperature to 20 ± 5 C stirring 2 - 3 hours, centrifugal to the solvent-free outflow, filter cake using mixed solvent (ethyl acetate 4.76 kg hexane 7.34 kg) leaching, centrifugal then adding to the solvent-free outflow. The centrifugal throughout the batch of wet product at the vacuum degree of - 0.08 - - 0.1 mpa lower, control temperature 40 - 50 C decompression drying 6 - 10 hours. A gray solid 6.00 kg compound (II). HPLC purity: 96.1%, yield: 73.4%. |
|
|
EXAMPLE 2PREPARATION OF BENZYL POSACONAZOLE OF FORMULA (Ila); In a clean, dry round bottomed flask 23 ml of dimethylsulfoxide and 4.74 gm of compound of formula (III) were charged at room temperature and stirred for about 15 minutes. Previously prepared NaOH solution (0.53 gm of NaOH dissolved in 3.74 ml of water) was added into the flask at about room temperature and stirred for about 30 minutes. 5 gm of compound of formula (IVa) was added to the reaction solution and stirred at about 35C to about 40C for about 12 hours. After completion of the reaction, the reaction solution was cooled to about 0C and 50 ml of water was added dropwise and stirred for about 30 minutes. The formed precipitate was filtered and washed with 80 ml of water. The solid was dried in air oven at about 45C to about 50C to yield 6.6 gm of the title compound. Purity by HPLC: 92% |
| 69.29 g |
With sodium hydroxide; In water; dimethyl sulfoxide; at 25 - 43℃; for 10h; |
Example-20: Preparation of 4-(4-(4-(4-(((3R,5R)-5-((1H-i,2,4-triazol-1-yi)methyl)-5-(2,4- difluorophenyl)tetrahydrofuran-3-yI)methoxy)phenyl)piperazin-1-yI)phenyl)-1-((2S,3S)-2-(benzyloxy)pentan-3-yI)-1H-1,2,4-triazol-5(4H)-oneSodium hydroxide solution [prepared by dissolving sodium hydroxide (7.8 gins) in water (10 ml)] was added to dimethyl sulfoxide (175 ml) at 25-30C. ((3S,5R)-5-((1H-1,2,4-triazol-1- yl)methyl)-5-(2,4-difluorophenyl)tetrahydrofuran-3-yl)methyl-4-methylbenzene sulfonate (48.12 gms) and then followed by 1 -((2S,3 S)-2-(benzyloxy)pentan-3-yl)-4-(4-(4-(4-hydroxyphenyl)piperazin- 1 -yl)phenyl)- 1 H-i ,2,4-triazol-5(4H)-one (50.Ogms) was added to the reaction mixtureat 25-30C. Heated the reaction mixture to 38-43C and stirred for 10 hours at the same temperature. Cooled the reaction mixture to 25-30C and slowly added to water at the same the same temperature. Cooled the reaction mixture to 10-15C and adjusted the pH of the reaction mixture to 7.0 using hydrochloric acid solution at the same temperature. Raised the temperature5 of the reaction mixture to 25-30C and stirred for 3 hours at the same temperature. Filtered the precipitated silid and washed with purified water. To the obtained wet compound, isopropanol (500 ml) was added at 25-30C. Heated the reaction mixture to 65-70C to get clear solution. Cooled the reaction mixture to 25-30C and stirred for 4 hours at the same temperature. Filtered the precipitated solid, washed with isopropanol and dried to get the title compound.10 Yield: 69.29 gins. |
| 13.2 g |
|
10 g of compound of formula A and 76 mL DMSO were added into a reaction flask at room temperature, and stirred until clarification. An alkaline solution previously prepared from 1.4 g sodium hydroxide and 6 g water was further added thereto. The mixture was stirred for 1 h. Then, 10 g of compound of formula IX was added thereto. The mixture was warmed up to 38 C. and reacted for 16 h. After the reaction was completed, the mixture was warmed up to 45 C. 6 mL water was added thereto. The mixture was stirred for 30-60 min. 154 mL water was further added thereto. The mixture was stirred for 1 h, and filtered under suction. The resultant solid was drip washed with 4×50 mL purified water to obtain a wet product. (0249) The wet product and 60 mL of 90% aqueous ethanol solution were added to a reaction flask, warmed up to 65 C., and stirred. After clarification, 1.5 g active carbon was added thereto. The mixture was stirred for 15 min, filter-pressed while hot, and washed with 15 mL of 90% aqueous ethanol solution. Then, the filtrate was warmed up to 65 C., stirred, cooled to 40-45 C. after clarification, crystallized while keeping the temperature for 1 h, further cooled to 0-5 C., crystallized while keeping the temperature for 1 h, and filtered under suction. The resultant solid was first drip washed with 5 mL of 50% aqueous ethanol solution, and then drip washed with 4×50 mL purified water. The solid was collected, placed in a ventilated oven at 50-55 C. and dried for 16 h to obtain 13.2 g of product. |
| 1.3 kg |
|
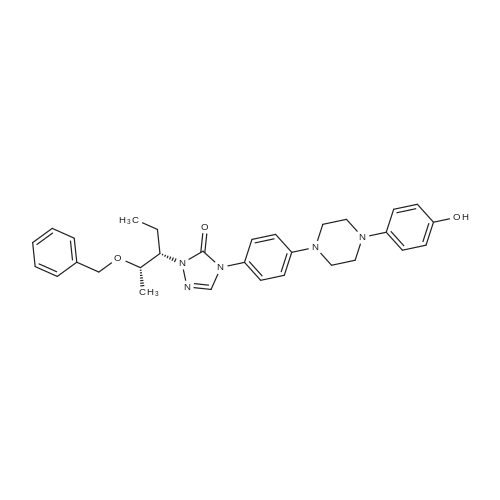
2-[(l S2S)-l-Ethyl-2-benzyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-l- piperozinyl] phenyl]-3H-l,2,4-triazol-3-one (1 kg) was added to the Flask along with Dimethylsulfoxide (8 lit) at room temperature and stirred for 15 min. Solution of Sodium hydroxide (0.15 kg) in Water (0.3 lit) was added at same temperature and maintained for 1 hr. ((3S,5R)-5-((lH-l,2,4-triazol-l-yl)methyl)-5-(2,4- difluorophenyl)-tetrahydrofuran-3-yl)methyl-4-methylbenzene sulfonate (1.2 kg) was added and maintained for 4-5 hrs. Water (10 lit) was added to the reaction mixture and stirred for 15 min. Ethyl acetate (7.5 lit) was added and stirred for 15 min. Aqueous layer and Ethyl acetate layer were separated and aqueous layer was extracted with Ethyl acetate (3 lit). Aqueous layer and Ethyl acetate layer were separated and total aqueous layer was washed with Water (5 lit) and stirred for 15 min. Aqueous layer and Ethyl acetate layer were separated and Ethyl acetate layer was washed with brine solution. Aqueous layer and Ethyl acetate layer were separated and Ethyl acetate layer was dried over Sodium sulfate and distilled under vacuum at below 50C. The resultant crude was treated with Isopropyl alcohol (10 lit) and heated to 75-80C, maintained for material dissolved and treated with activated Carbon (0.05 kg) and maintained for 1 hr. The material was filtered through the Hyflow bed and washed with Isopropyl alcohol (1 lit). The resultant mass was cooled to room temperature, maintained for 2 hrs, filtered the solid and washed with Isopropyl alcohol (1 lit). Yield: 1.3Kg; HPLC: 98.6%. |
| 19.2 kg |
|
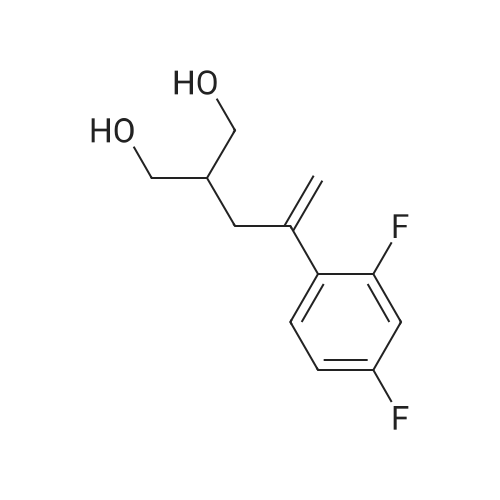
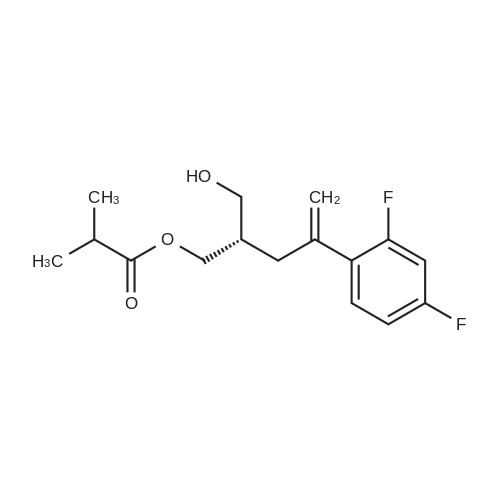
In a dry 300 L reactor, 70 kg of tetrahydrofuran was added under vacuum. The mixture was stirred, and after nitrogen replacement, the temperature was lowered to -10 C, and then 0.6 kg of sodium hydride was added in portions under nitrogen protection, and after stirring for 0.5 h, Continue to control the temperature T ? -10 C to add 12 kg of POB/24 kg of tetrahydrofuran solution to the system, After the dropwise addition, the mixture was stirred for 1 hour. Add 10 kg of POA/20 kg of tetrahydrofuran solution to the system. The reaction was carried out at a temperature of -10 to 0 C, and the monitoring until the POA disappeared. The temperature was controlled by T ? -10 C, and 1 kg of methanol was added dropwise. After stirring for 0.5 h, the reaction was quenched by adding 1 kg of water. Temperature control T ? 40 C under reduced pressure to concentrate about 70 L of tetrahydrofuran, Add 200 kg of dichloromethane and wash with 50 kg * 3 tap water. Wash with 50 kg of saturated brine, and dry the organic phase with 10 kg of anhydrous sodium sulfate. Filtration, temperature control T ? 40 C, concentrated under reduced pressure to no fraction, 60 kg of ethyl acetate was added while hot, and the temperature was raised to 70 C for 1 h. Then, the temperature is lowered to 0 to 10 C at a rate of 10 C / h, and suction filtration is performed. The filter cake was rinsed with pre-cooled 5 kg of ethyl acetate and drained. Transfer to the drying room at 40 ~ 45 C under reduced pressure for 40h, Received 19.2 kg of POC. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +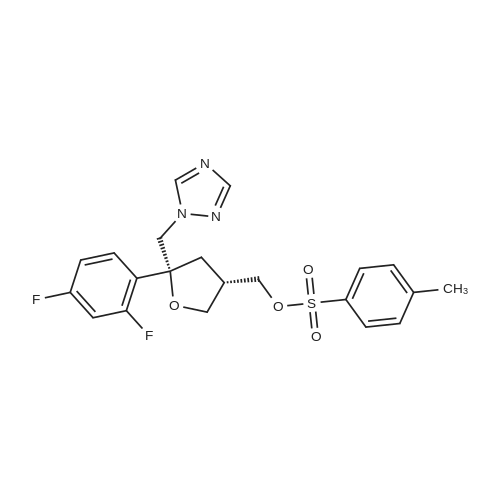

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping