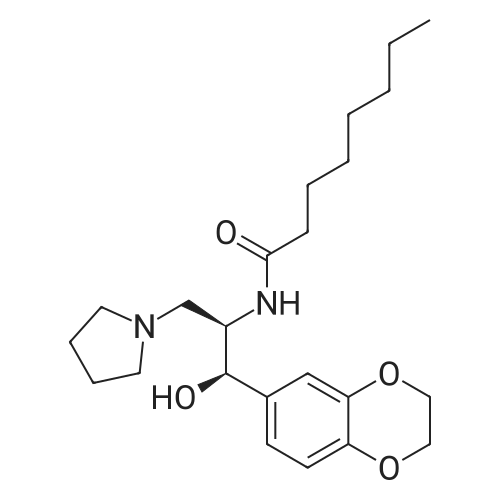
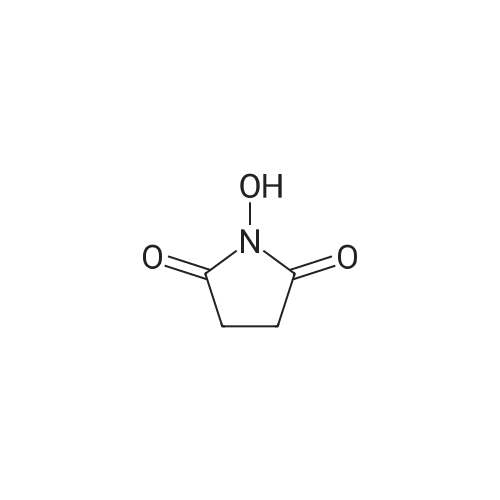
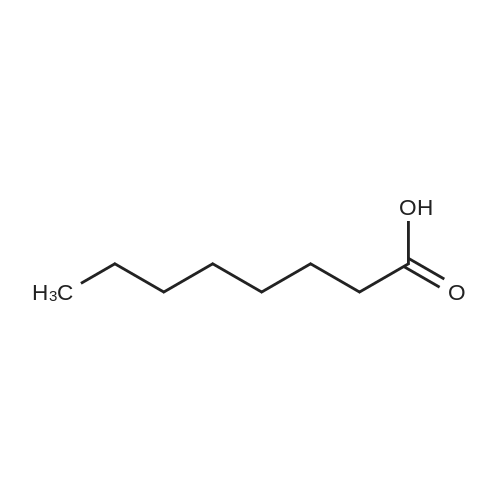
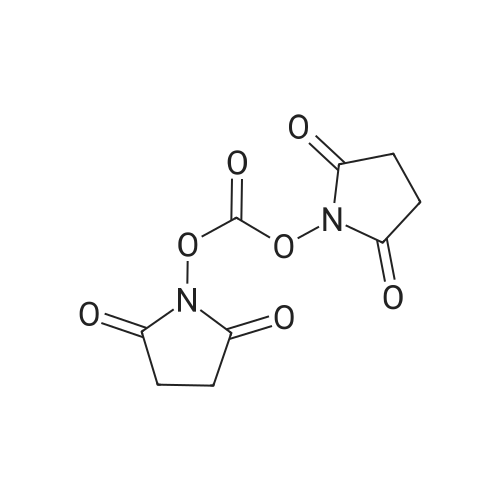
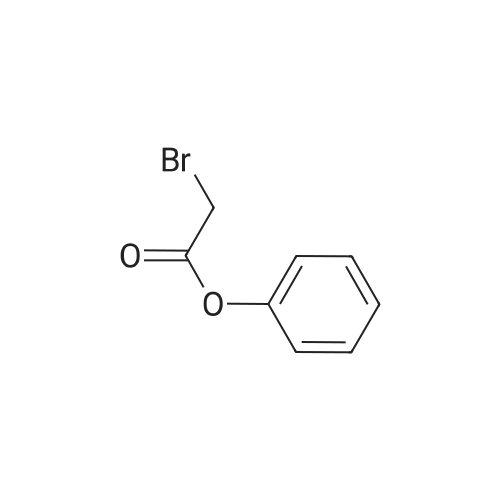
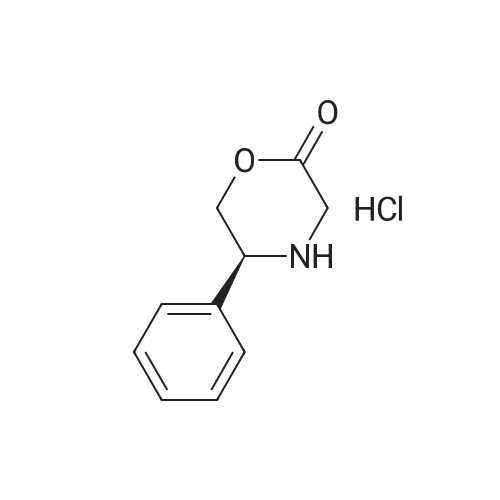
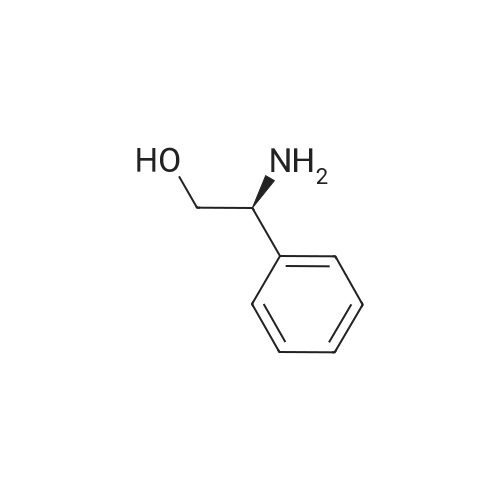
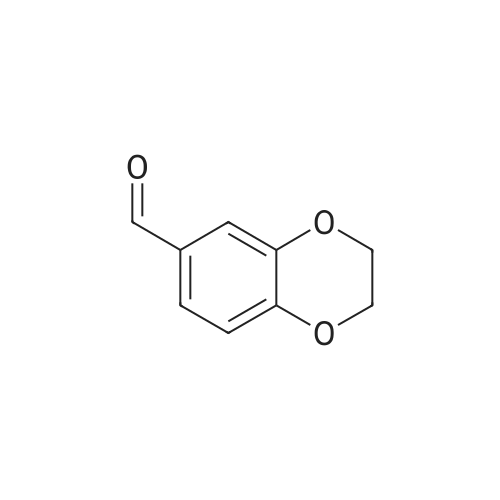
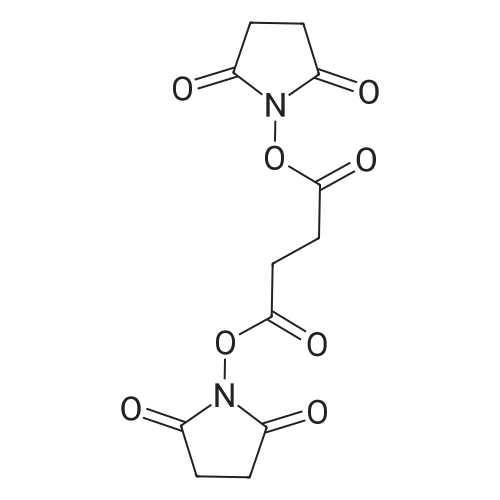
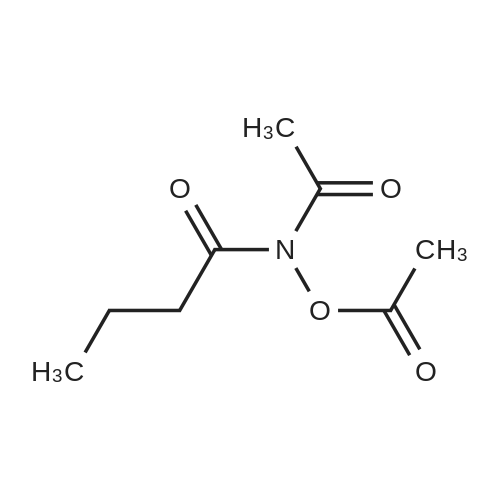
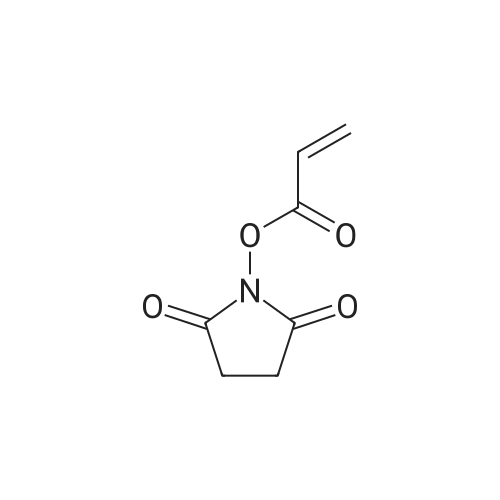
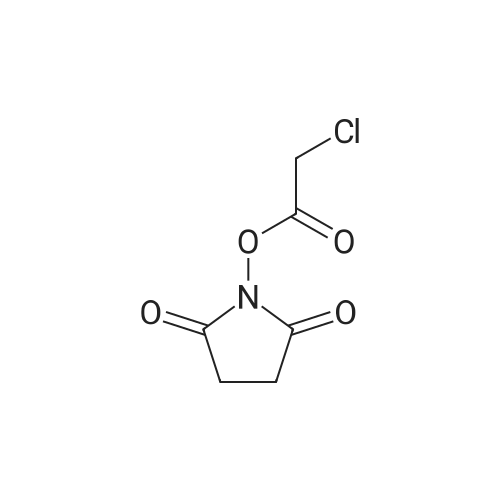
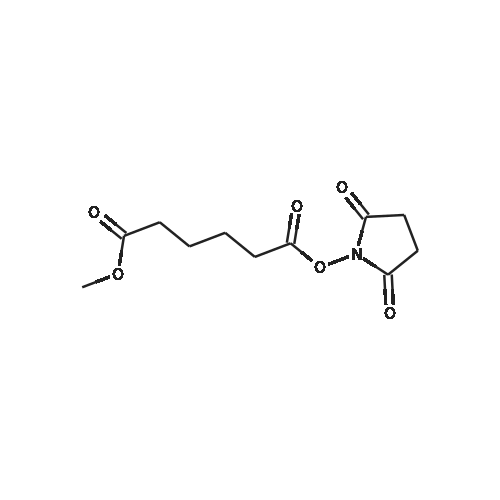
| 55% |
In dichloromethane; at 20℃; for 20h;Inert atmosphere; |
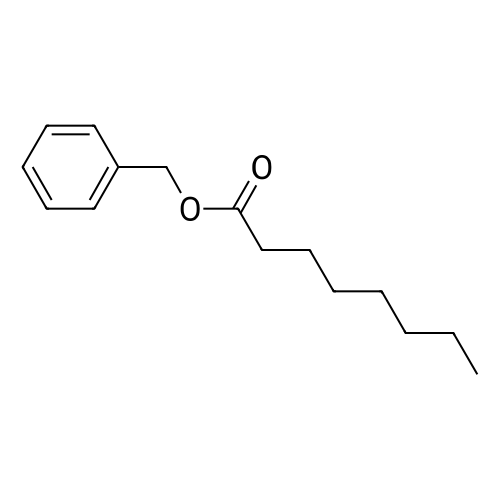
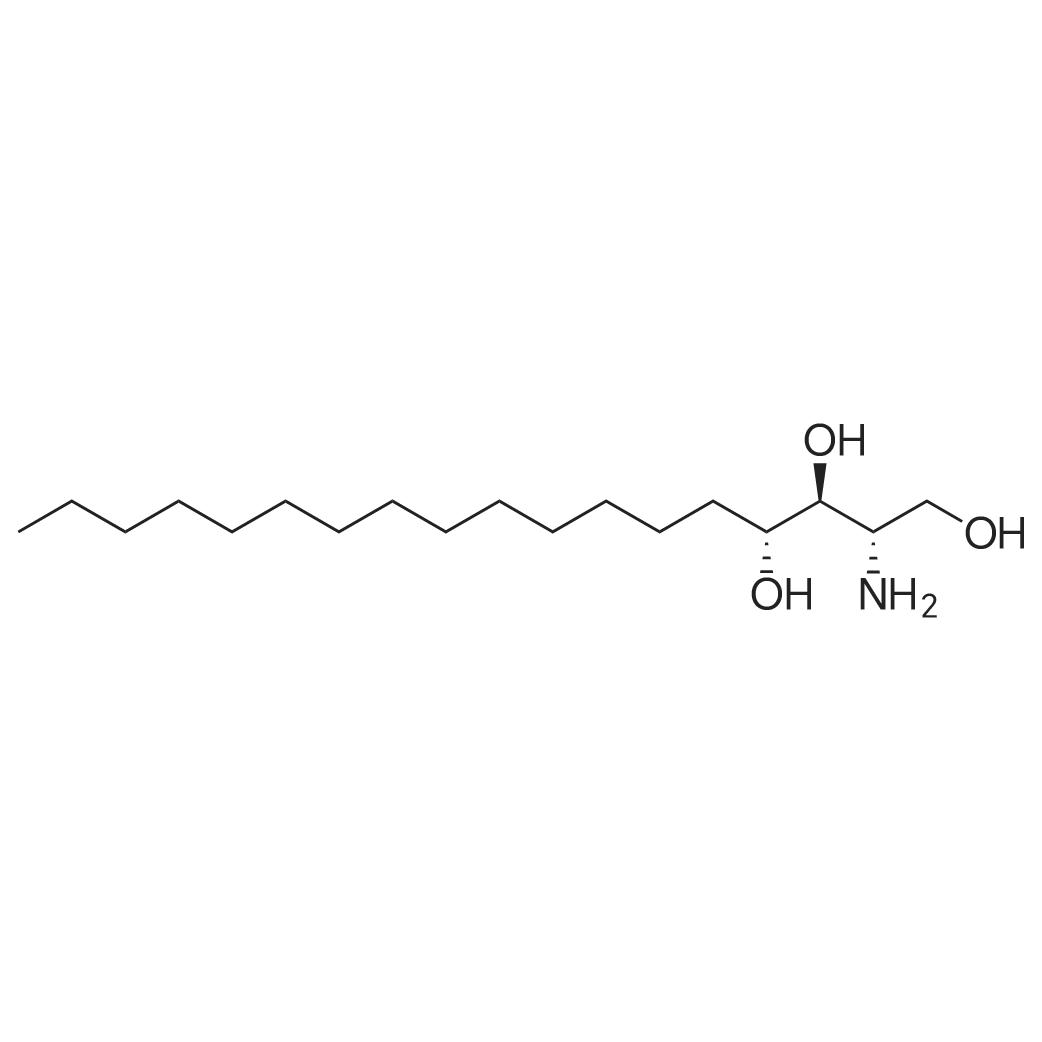
At 25 ~ 30 C, the raw material EGS-SMA2.5g (1 eq) was dissolved in 50 ml of anhydrous DCM and the raw material EGS-SMB2.2g (1 eq) was dissolved in 25 ml of anhydrous DCM and replaced with nitrogen twice. Under nitrogen, the EGS-SMA solution was slowly added dropwise to the EGS-SMB solution at room temperature for 30 min. After the addition was completed, the reaction was continued for 20 hours at room temperature. 35 ml of 1 M NaOH was added to the reaction system and stirred for 45 min. After the completion of the reaction, the reaction system was separated, the organic phase was washed twice with 20 ml of 1 M NaOH, washed twice, and dried to dryness to obtain a viscous yellow liquid. And further adding 100 ml of a 5% EA n-hexane solution to the reaction system, heating and refluxing to dissolve most of the crude product, cooling to 40 C, and pouring the liquid into another reaction flask. The remaining yellow viscous yellow liquid was further added to 20 ml of a 5% EA solution of n-hexane, heated to reflux to dissolve most of the crude product, cooled to 40 C, poured out and repeated. The resulting n-hexane solution was stirred at room temperature for 4 h, filtered, and 10 ml of 5% EA of n-hexane was washed twice to give 2 g of a white product as EGS-API in 55% yield. |
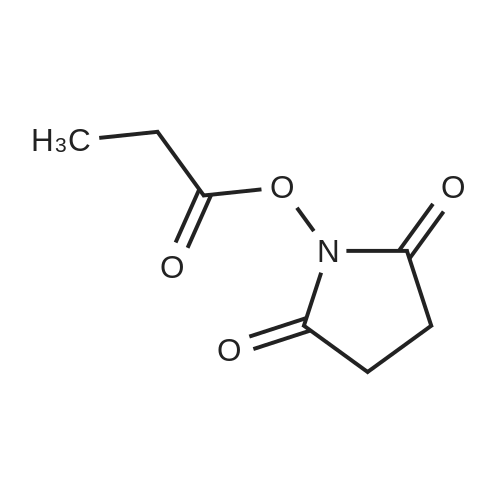
| 39% |
In dichloromethane; at 20℃;Inert atmosphere; |
Compound 6 (1R,2R)-Octanoic acid [2-(2',3'-dihydro-benzo[1,4]dioxin-6'-yl)-2-hydroxy-1-pyrrolidin-1-ylmethyl-ethyl]-amide To Intermediate 5 (22.36 g, 80.33 mmol) dissolved in anhydrous methylene chloride (300 mL) was added a solution of octanoic acid N-hydroxysuccinimide ester (19.4 g, 80.39 mmol) dissolved in anhydrous methylene chloride (150 mL) over 15-30 minutes under nitrogen at room temperature. The solution was stirred at room temperature for 18-20 hours. To the reaction was added 1M aqueous NaOH solution (200 mL). The two phase system was stirred at room temperature for 45 minutes. The layers were separated and the combined organic layers were washed twice with 1M NaOH (2*200 mL) and twice with water (2*100 mL). The organic layer was dried with sodium sulfate, filtered and rotoevaporated to a yellow oil. Most of the crude material was dissolved in 5% ethyl acetate in heptane (1 L) at reflux. After cooling to 40 C., the hazy solution was separated from the yellow oil by decanting the solution into a new flask. The first flask was rinsed twice with 5% ethyl acetate in heptane (2*250 mL) by the same process (reflux and cooling to 40 C. and decanting the solution from the oil). The combined solution was heated to reflux and allowed to cool to room temperature over 4 hours. The resulting white solid was filtered and washed with 5% ethyl acetate in heptane (100 mL) and heptane (100 mL). The white solid (13.9 g) was dried under vacuum for 16-24 hours. This solid was mostly dissolved in 5% ethyl acetate in heptane (800 mL) at reflux. After cooling to 50 C., the hazy solution was separated from the yellow oil by decanting the solution into a new flask. The first flask was rinsed with 5% ethyl acetate in heptane (100 mL) by the same process (reflux and cooling to 50 C. and decanting the solution from the oil). The combined solution was heated to reflux and allowed to cool to room temperature over 4 hours. The resulting white solid was filtered and washed with 5% ethyl acetate/heptane (50 mL) and heptane (50 mL). After drying at room temperature under vacuum for 2-3 days, Compound 6 was obtained in 39% yield (12.57 g). Analytical chiral HPLC (column: Chirex (S)-VAL and (R)-NE, 4.6*250 mm) showed this material to be 99.9% the desired R,R isomer. Analytical HPLC showed this material to be 99.6% pure. mp 87-88 C. 1H NMR (CDCl3) delta 6.86-6.73 (m, 3H), 5.84 (d, J=7.3 Hz, 1H), 4.91 (d, J=3.4 Hz, 1H), 4.25 (s, 4H), 4.24-4.18 (m, 1H), 2.85-2.75 (m, 2H), 2.69-2.62 (m, 4H), 2.10 (t, J=7.3 Hz, 2H), 1.55-1.45 (m, 2H), 1.70-1.85 (m, 4H), 1.30-1.15 (m, 8H), 0.87 (t, J=6.9 Hz, 3H) ppm. |
|
In toluene; at 52 - 54℃; for 4.5h; |
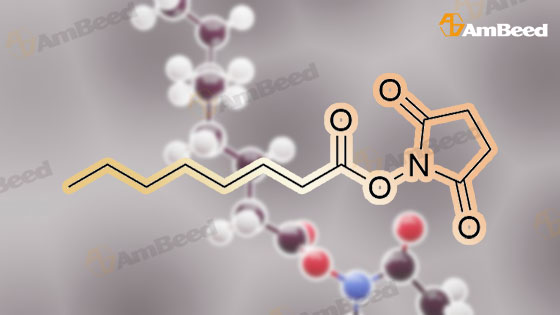
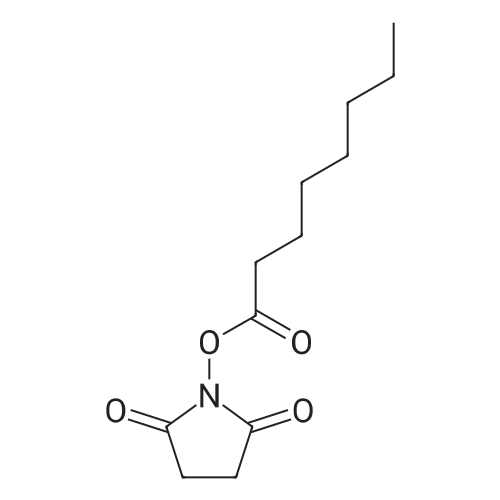
A flask was charged with (1 R,2R)-2-amino-1 -(2,3-dihydrobenzo[b][1 ,4]dioxin-6-yl)-3- (pyrrolidin-1-yl)propan-1-ol (82 g) in toluene (15 mL) and heated to about 52-54C followed by addition of 2,5-dioxopyrrolidin-1-yl octanoate (71.lg). The mixture was maintained at the same temperature for about 4.5 hours, completion of the reaction is monitored by TLC. Then the mixture was cooled to about 15C at which point 1M sodium hydroxide solution (410 mL) was slowly added over a period of 15 minutes and mixture was stirred at about 28C for another 15-20 minutes. The organic layer was separated and washed with 10% aqueous sodium chloride (2x492 mL), then subjected to vacuum distillation at below 55C to afford the crude compound. Then 20% MTBE in n-hexane (656 mL) was added to the crude material and mixture was stirred at 26-28C for about 3.5 hours followed by filtration of the solid and its washing with n-hexane (246 mL). The obtained solid was dried under vacuum at about 40C for 4.5 hours to afford the title compound. |
| 0.3 g |
With N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 25℃; for 24h; |
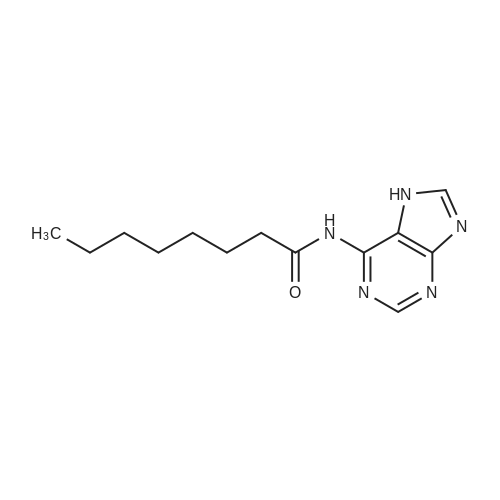
1 mmol of compound VIII (wherein R5 is a hydrogen atom) was dissolved in 30 ml of DMF, 1.5 mmol of DIPEA was added1.2 mmol of compound IX (wherein R6 is succinimidoyl). Plus 25, 24 hours under the reaction. After the reaction, add water quenchingAnd extracted twice with ethyl acetate. The combined organic phase was washed and dried and concentrated to give 0.3g of etilogluzide |
| 160 mg |
With N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 18h; |
Without furher purification, 7 (150 mg, 0.55 mmol) was dissolved in DMF (5 mL), DIPEA (0.16 mL, 0.95 mmol) was added to the mixture. The reaction was stirred for 5 min, then 2,5-dioxopyrrolidin-1-yl octanoate (150 mg, 0.6 mmol) was added and the mixture was stirred at room temperature for 18 h. Water was added to the reaction (15 mL) and extracted with EtOAc (3×30 mL). The combined organics were washed with brine, dried over Na2SO4, filtered, concentrated. The residue was purified by flash chromatography (EtOAc/ petroleum ether, 1:10 to DCM/MeOH, 10:1) to afford 1 (160 mg, 61% for two steps) as a white solid. mp 85-87C; [alpha]eq o(sup 6(20),sdo 2( D))20 D +13 (c, 0.23, CHCl3); 1H NMR (400 MHz, CDCl3) delta 6.76-6.85 (m, 3H), 5.83 (d, J = 7.2 Hz, 1H), 4.90 (d, J = 3.6 Hz, 1H), 4.24 (s, 4H), 4.16-4.20 (m, 1H), 2.74-2.84 (m, 2H), 2.62-2.67 (m, 4H), 2.08-2.12 (m, 2H), 1.77-1.80 (m, 4H), 1.52-1.55 (m, 2H), 1.20-1.30 (m, 10H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (150 MHz, CDCl3) delta 173.4, 143.4, 142.8, 134.4, 118.9, 117.0, 115.0, 75.5, 64.3, 57.8, 55.2, 52.2, 36.8, 31.6, 29.1, 29.0, 25.6, 23.6, 22.6, 14.1. HR-MS (ESI) calcd for C23H37O4 N2 (M+H)+: 405.2748, found 405.2725. [1] |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping