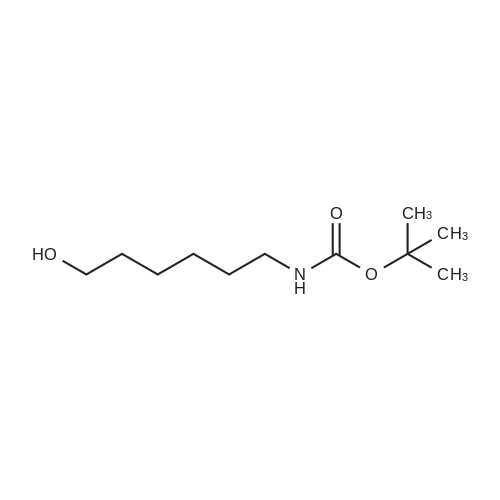
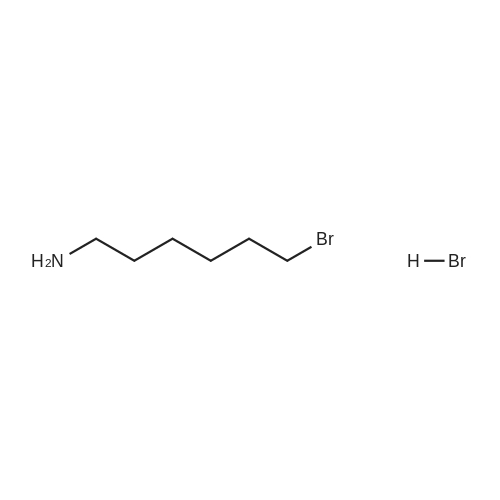
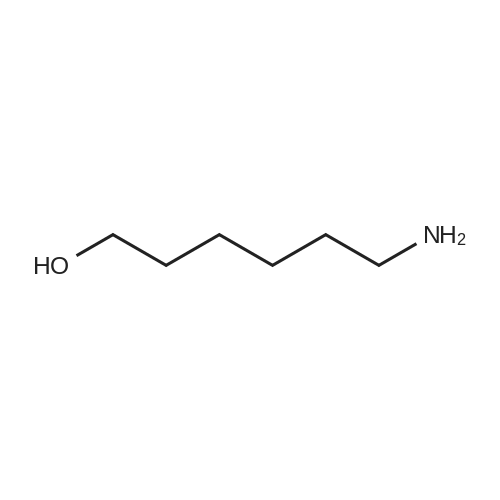
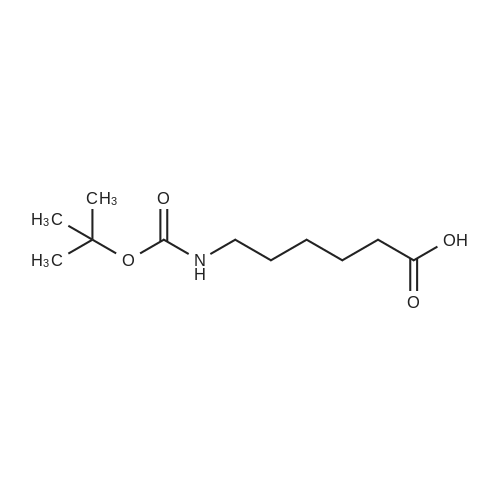
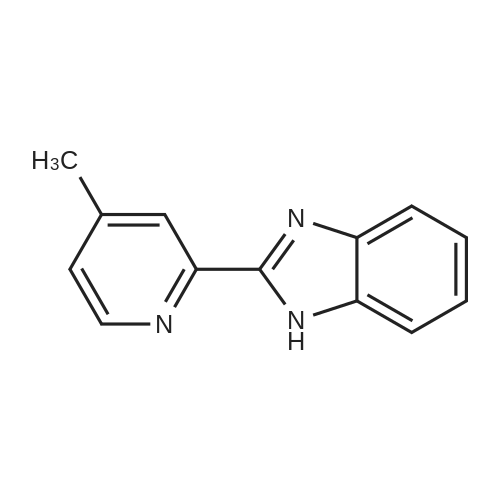
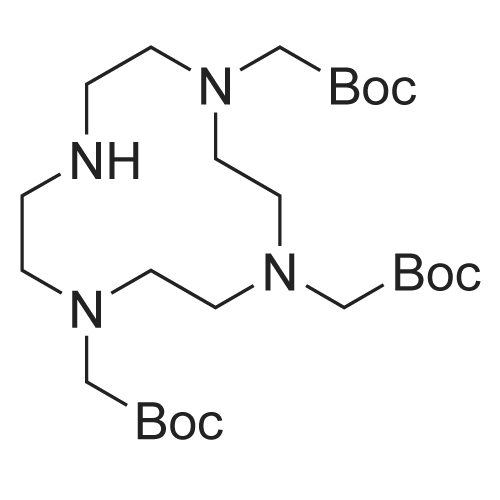
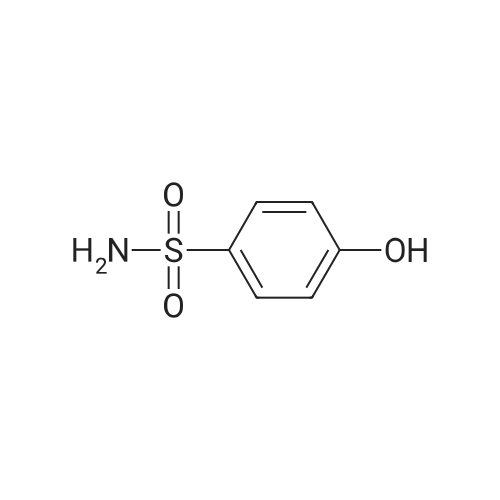
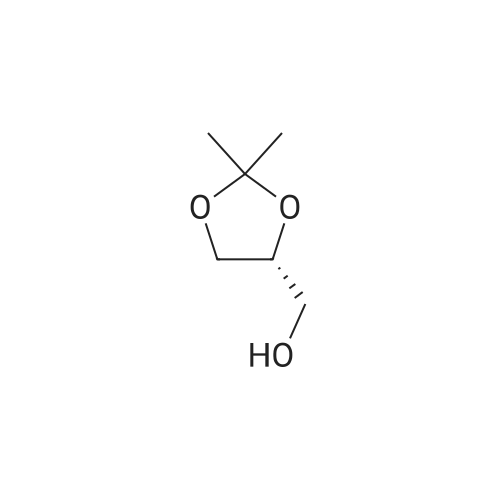
| 13% |
|
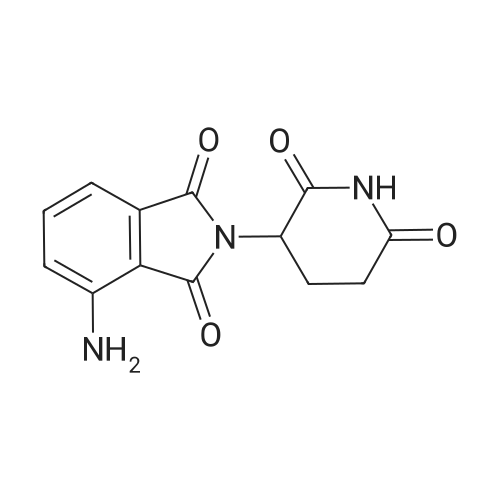
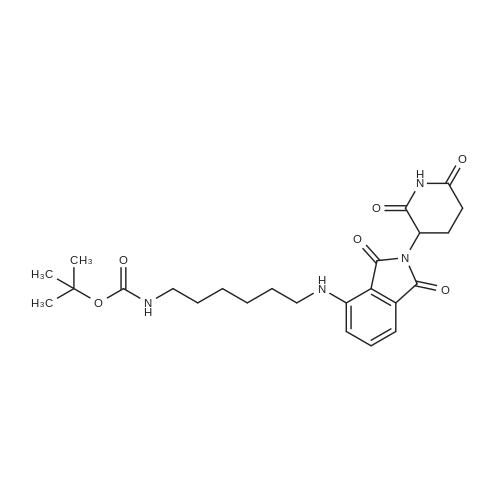
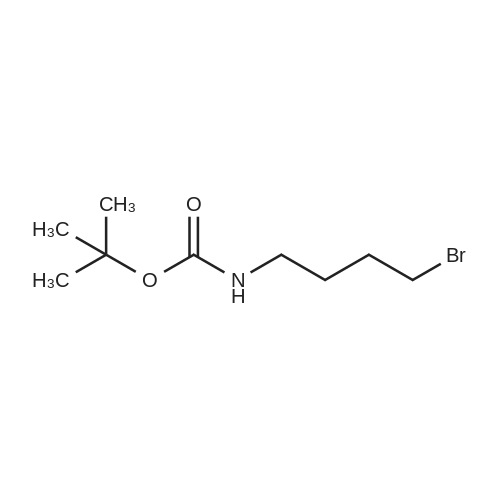
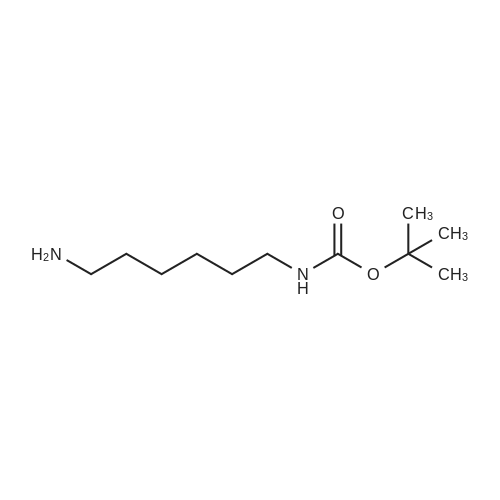
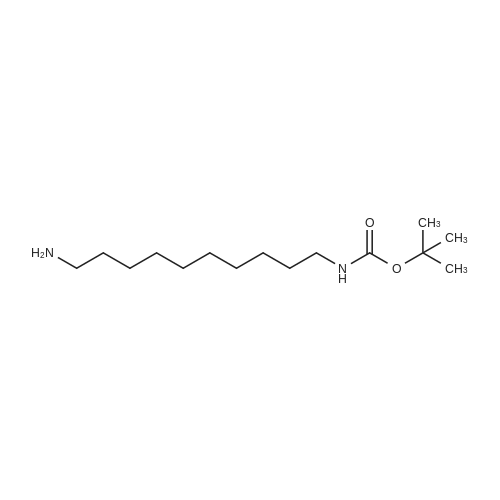
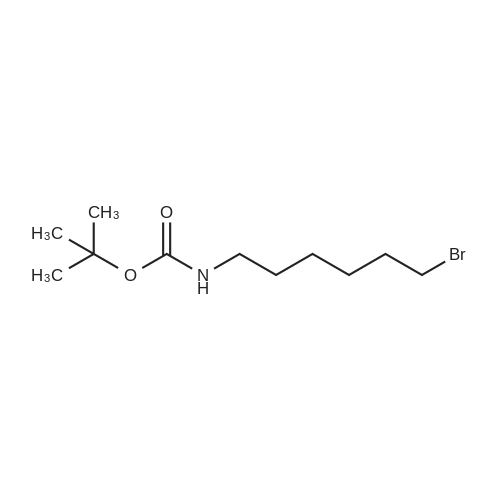
To a suspension of N-{4-[5-(1H-indazol-5-yl)-6-oxo-1-(2,2,2-trifluoroethyl)-1,6- dihydropyridazin-3-yl]phenyl}-1,3-dihydro-2H-pyrrolo[3,4-c]pyridine-2-carboxamide hydrogen chloride (see Example 22, raw product before HPLC purification was used, 100 mg, 176 mumol) in DMF (1.9 mL) was added at 0C sodium hydride (14.8 mg, 60% in mineral oil, 370 mumol) under an argon atmosphere. The mixture was stirred for 30 min at that temperature. Then tetra-n-butylammonium iodide (6.50 mg, 17.6 mumol) and tert-butyl (6- bromohexyl)carbamate (41 mul, 180 mumol) were added. After stirring for additional 2 h at 0C the mixture was was diluted with aqueous ammonium chloride solution, and directly purified by preparative HPLC to yield 24.3 mg (80% purity, 15% yield) of the title compound and 17.4 mg (95% purity, 13% yield) of tert-butyl(6-{5-[6-{4-[(1,3-dihydro-2H-pyrrolo[3,4-c]pyridin-2- ylcarbonyl)amino]phenyl}-3-oxo-2-(2,2,2-trifluoroethyl)-2,3-dihydropyridazin-4-yl]-2H-indazol- 2-yl}hexyl)carbamate as a second product. (1392) HPLC: Instrument: Labomatic HD-5000, pump head HDK-280, gradient module NDB-1000, fraction collector Labomatic Labocol Vario 2000, Knauer UV detector Azura UVD 2.1S, Prepcon 5 software. Column: Chromatorex C18 10mum 125x30 mm. Eluent A: water + 0.1% ammonia; Eluent B: acetonitrile; gradient: 0-6 min 40-80% B, 6-8 min 80-100% B, rate 150 mL/min, temperature 25C. LC-MS (Method 2): Rt = 1.31 min; MS (ESIpos): m/z = 731[M+H]+. (1393) 1H-NMR (400 MHz, DMSO-d6) delta [ppm]: 1.231 (1.07), 1.256 (0.81), 1.275 (0.72), 1.324 (0.69), 1.353 (16.00), 1.366 (6.83), 1.793 (0.32), 1.812 (0.41), 1.830 (0.54), 1.848 (0.38), 2.326 (0.92), 2.331 (0.75), 2.664 (0.68), 2.668 (0.95), 2.673 (0.78), 2.848 (0.64), 2.863 (0.78), 2.879 (0.64), 2.893 (0.43), 3.500 (0.38), 3.516 (0.80), 3.533 (0.40), 4.431 (0.46), 4.447 (0.92), 4.464 (0.48), 4.834 (1.07), 4.852 (1.11), 5.114 (0.55), 5.137 (0.54), 6.752 (0.38), 7.443 (0.54), 7.456 (0.57), 7.742 (1.07), 7.764 (1.43), 7.791 (0.66), 7.939 (1.24), 7.948 (0.84), 7.951 (0.83), 7.961 (1.15), 7.970 (0.60), 7.974 (0.60), 8.194 (1.49), 8.240 (1.41), 8.474 (0.98), 8.505 (0.69), 8.517 (0.68), 8.624 (1.06), 8.682 (0.92). (1394) Second product: tert-Butyl(6-{5-[6-{4-[(1,3-dihydro-2H-pyrrolo[3,4-c]pyridin-2- ylcarbonyl)amino] phenyl}-3-oxo-2-(2,2,2-trifluoroethyl)-2,3-dihydropyridazin-4-yl]-2H-indazol- 2-yl}hexyl) carbamate (1395) LC-MS (Method 2): Rt = 1.26 min; MS (ESIpos): m/z = 731[M+H]+. (1396) 1H-NMR (400 MHz, DMSO-d6) delta [ppm]: 1.200 (0.21), 1.231 (0.45), 1.254 (0.37), 1.272 (0.30), 1.294 (0.27), 1.309 (0.28), 1.323 (0.34), 1.341 (0.43), 1.359 (16.00), 1.905 (0.26), 1.922 (0.37), 1.940 (0.28), 2.518 (3.00), 2.523 (2.43), 2.539 (0.49), 2.848 (0.20), 2.865 (0.45), 2.880 (0.47), 2.897 (0.18), 4.429 (0.33), 4.446 (0.70), 4.463 (0.35), 4.833 (0.80), 4.851 (0.83), 5.109 (0.39), 5.132 (0.39), 6.766 (0.26), 7.442 (0.45), 7.454 (0.48), 7.671 (0.45), 7.694 (0.66), 7.740 (0.99), 7.763 (1.53), 7.768 (0.78), 7.786 (0.39), 7.791 (0.40), 7.934 (1.18), 7.957 (0.95), 8.207 (1.37), 8.483 (0.76), 8.485 (0.75), 8.487 (0.65), 8.504 (0.71), 8.517 (0.66), 8.548 (1.06), 8.623 (0.91), 8.681 (0.80). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping