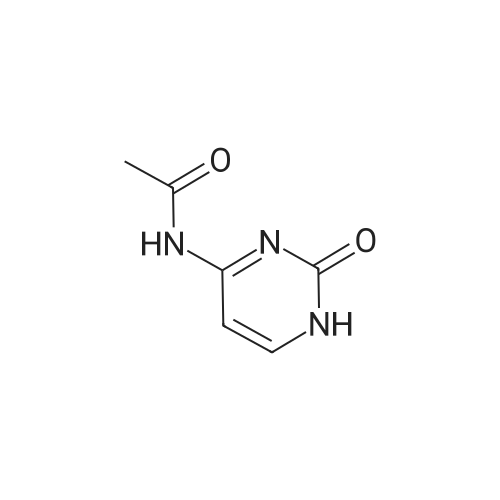
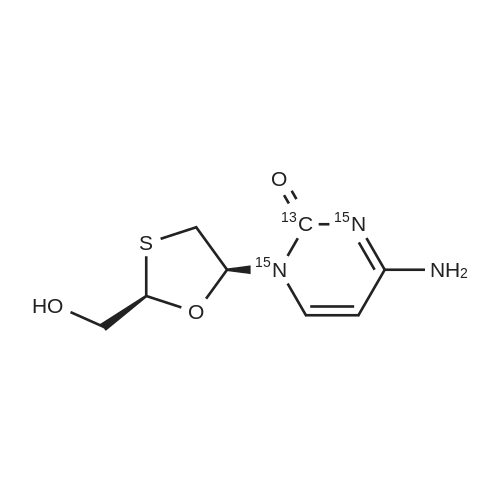
| 90% |
Stage #1: 4-amino-1-[(2R,5S)-(2-hydroxymethyl)-1,3-oxathiolan-5-yl]-1,2-dihydropyrimidin-2-one (S)-BINOL co-crystal With hydrogenchloride In water; ethyl acetate at 20℃; for 1h;
Stage #2: With sodium hydroxide In water |
6; 2
Charged (S) BINOL Cocrystal (20g) in ethyl acetate (100ml) and D.M. water (100ml) at RT. Cone. Hydrochloric acid (4 to 5 ml) was added to the mixture gradually and stirred (pH of the mixture was between about 3 to 4) for an hour and allowed the layers to settle, separated the layers. Aqueous layer was washed with fresh ethyl acetate to remove S(-) BINOL completely. pH of the solution was adjusted to about 7 using 10% sodium hydroxide solution. The solution was then passed though activated resin 225-H column. The column was then washed with purified water, thereafter with 15% aqueous ammonia solution. Combined solutions were subjected to evaporation till 20 to 50 ml. To the mass was then added .8 ml of DNS and stirred to homogenize. The solution was then passed through 0.5 micron filter and then the solution was seeded with Lamivudine form I. Further, the mixture was cooled to around 8 - 10°C and stirred for an hour maintaining the same temperature. The crystalline product was then filtered and washed with precooled water and DNS mixture. The product was then dried under vacuum to afford Lamivudine form I.Results:Out put Yield (w/w) % Yield in HPLC purity (RS) Chiral purity by (Range) (Range) Range HPLC6.5-8 gm 0.30-0.40 68-90% Cis (-): 99.94 % Cis (-): 99.95%Individual Impurity: Cis (+): 0.06 % 0.03 % |
|
With hydrogenchloride In water; ethyl acetate |
9
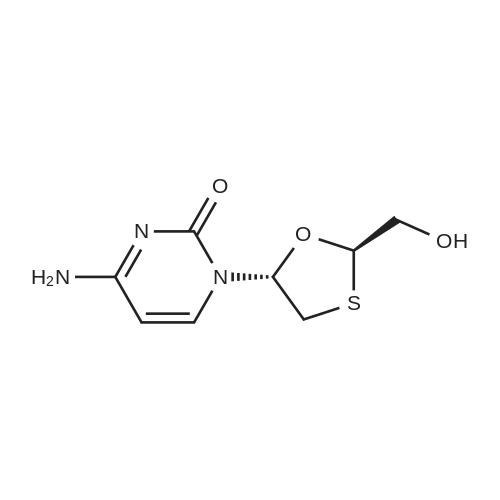
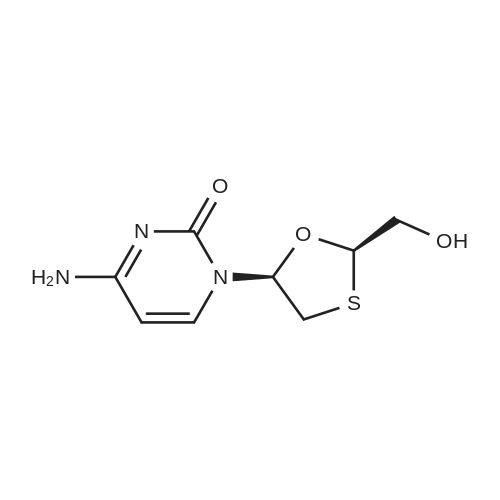
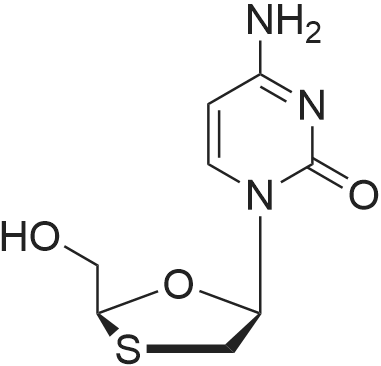
Preparation of Lamivudine: (-)-[2R,5S]-4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidin-2-oneCompound I5 mL of conc. HCl was slowly added to a solution of 20 g of Lamivudine-BINOL complex in 100 ml of ethylacetate and 100 mL of DM water (pH 2-2.5). The layers were separated and a 100 mL aliquot of ethylacetate was added to the aqueous layer. The layers were separated again and the aqueous layer was neutralized using 10 mL of 10% aqueous NaOH solution. The solvent was recovered under vacuum at 40-45° C., the product obtained was dissolved in 160 mL of methanol, filtered, the filtrate was concentrated and 32 mL of water-ethanol mixture (3:1) was added to this product, heated to get a clear solution, cooled to 5-10° C. and then filtered. The residue was vacuum dried at 45-50° C. Yield: 4-5 g.Enantiomeric excess=99.74%m.p.=133-135° C.[α]D at 25° C.=98.32° (c=5 water)1H NMR (DMSO d6): 2.99-3.07 (dd, 1H), 3.35-3.38 (dd, 1H), 3.72-3.74 (m, 2H), 5.14-5.18 (t, 1H), 5.32-5.38 (t, 1H), 5.71-5.75 (d, 1H), 6.16-6.21 (t, 1H), 7.22-7.27 (d, 2H), 7.80-7.83 (d, 1H)Moisture content: 1.67%IR (in KBr, cm-1): 3551, 3236, 2927, 1614, 1492, 1404, 1336, 1253, 1146, 1052, 967, 786.MS: M+1=230XRD [2θ] (Cu-Kα1=1.54060 , Kα2=1.54443 Kβ=1.39225 ; 40 mA, 45 kV): 5.08, 9.89, 10.16, 11.40, 11.65, 12.96, 13.23, 15.26, 15.82, 17.74, 18.74, 18.88, 19.67, 20.69, 22.13, 22.88, 23.71, 25.47, 26.07. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping