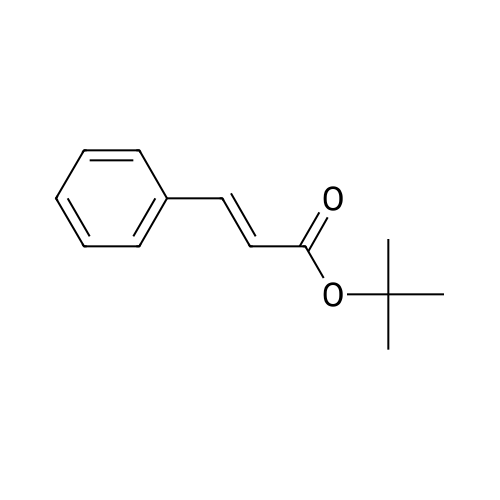
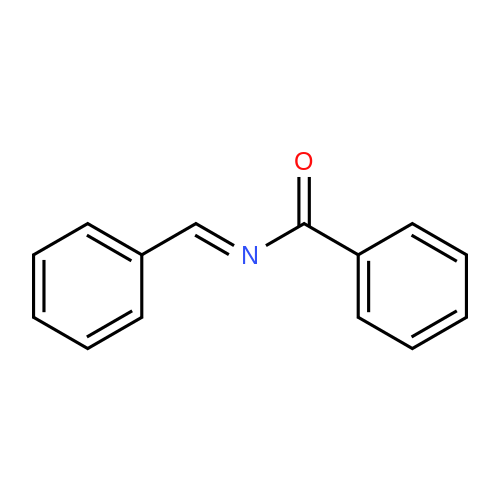
| 65% |
|
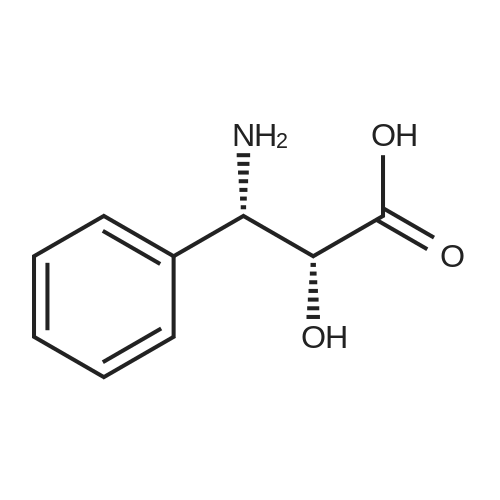
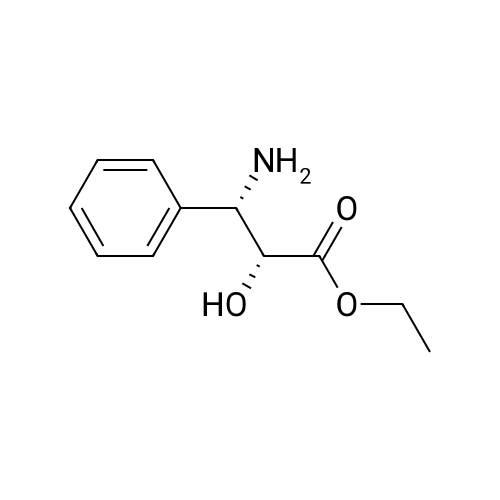
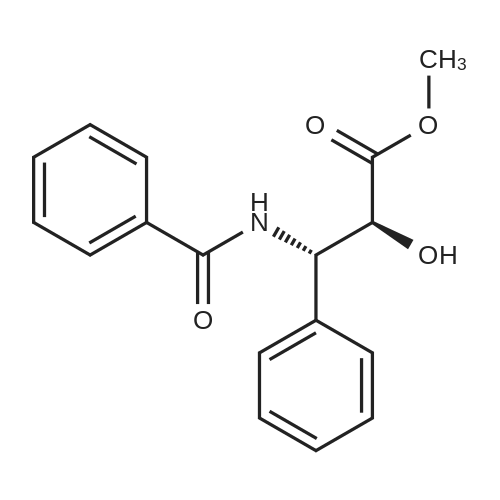
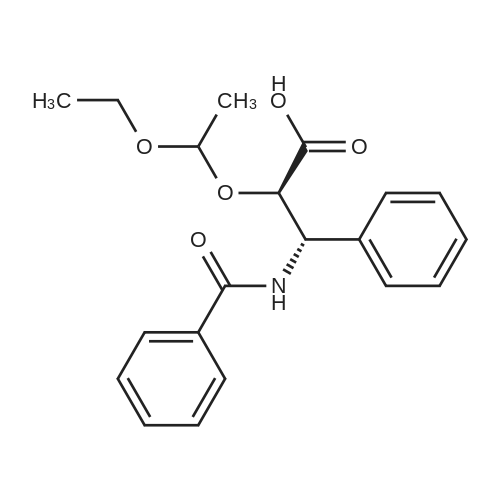
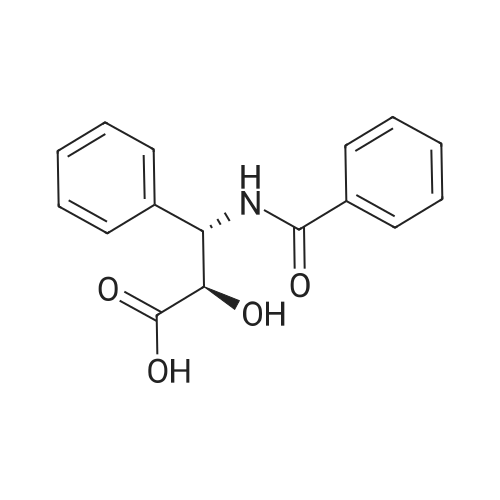
N-Benzoyl-(2R,3S)-2-hydroxy-3-amino-3-phenylpropionic Acid (4) STR18 Compound 4. The enantiomerically enriched 37 (0.43 g, 2.37 mmol) was converted to N-benzoyl-(2R,3S)-2-hydroxy-3-amino-3-phenylpropionic acid (4, 0.44 g, 65%) according to our earlier Schotten-Baumann-based procedure for this same transformation (Sharpless et al. J. Org. Chem. 59 (1994), 5104). Chemically and enantiomerically pure 4 was isolated by simple filtration of the solid which appeared when the pH of the reaction mixture was adjusted to ca. 2 by addition of aqueous HCl. m.p. 166-167 C. (lit: Ojima et al. J. Org. Chem 56 (1991) 1681: 167-169 C.); [a].backslash.o(25,D) -34.0 (c 0.50, EtOH) (lit: Sharpless et al. J. Org. Chem. 1976, 41, 177: [a].backslash.o(25,D) -35.9 c 0.565, EtOH); lit3d [a].backslash.o(25,D) -35.5 (c 1.07, EtOH); 1H NMR (400 MHz, DMSQ) delta4.37 (d, J=4.3 Hz, 1H), 5.46 (dd, J=8.8, 4.2 Hz, 1H), 7.22-7.55 (m, 9H), 7.84 (d, J=7.2 Hz, 1H), 8.60 (d, J=8.9 Hz, 1H), 12.73 (br, 1H); 13 C NMR (100 MHz, DMSO) delta173.5, 166.0, 140.3, 134.4, 131.4, 128.4, 128.0, 127.4, 127.2, 126.9, 73.6, 55.8. |
|
|
Synthesis of Compound 4 STR4 To a solution of NaOH (3.05 equivalents) in water (0.13 Molar; based on initial olefin concentration) was added ethyl carbamate (3.10 equivalents). The resulting solution was stirred at room temperature for 10 minutes and then t-butyl hypochlorite (3.05 equivalents; Aldrich Chemical Company) was added dropwise at 0 C. The above solution was stirred for another 10 minutes and then n-propanol (0.13 Molar; based on initial olefin concentration) and (DHQ)2 -PHAL (5 mol %) were added to form a homogeneous solution. The reaction mixture was immersed in a room temperature bath and then cyclohexene (1 equivalent; Aldrich) and K2 OsO2 (OH)4 (4 mol %) were then added. The reaction was stirred for 45 minutes at room temperature with the color changing from light green to light yellow, followed by quenching by addition of aqueous sodium sulfite (saturated 0.17 Molar; based on initial olefin concentration). The phases were separated, and the aqueous phase was extracted with ethyl acetate (3*0.20 M volumes). The combined organic extracts were washed with water and brine, dried over MgSO4 and the solvent was concentrated to give the crude product, which also contained the ethyl carbamate by-product produced upon the reduction of the excess N-chlora-N-sodiocarbamate. Flash chromatography (6:4:1 hexane/CHCl3 /MeOH) of this material provided vicinal hydroxycarbamate product. If benzyl carbamate was used, an additional 0.25 M volume of n-propanol was added with water to dissolve all of benzyl carbamate before adding t-butyl hypochlorite. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping