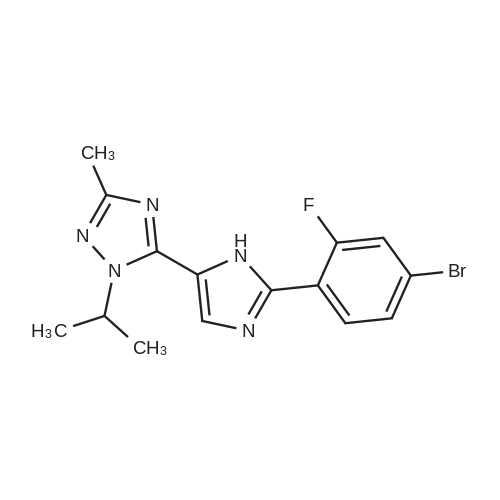
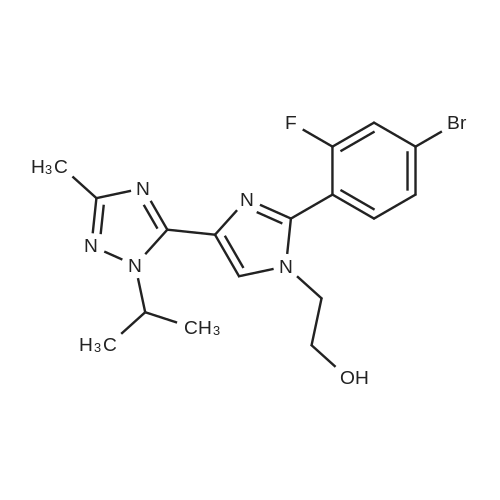
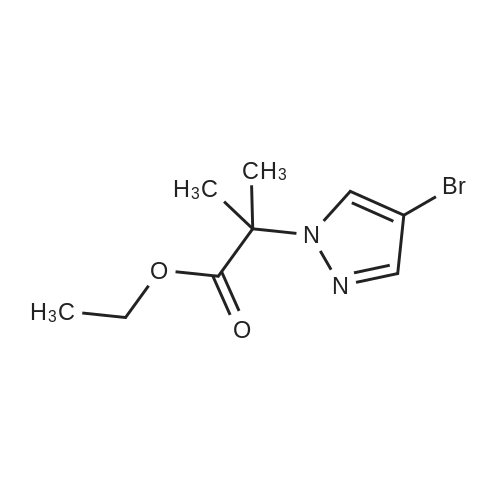
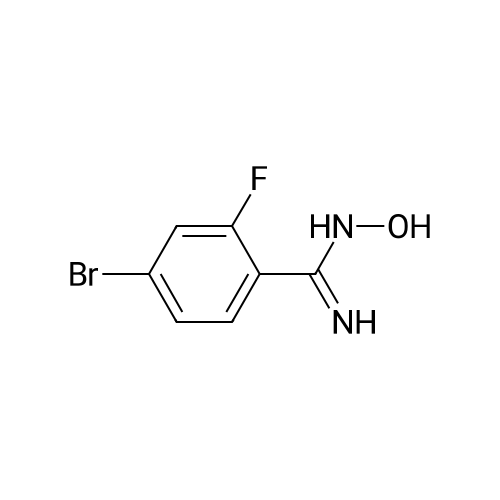
| 82% |
With ammonium chloride; N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; for 2h; |
196.3
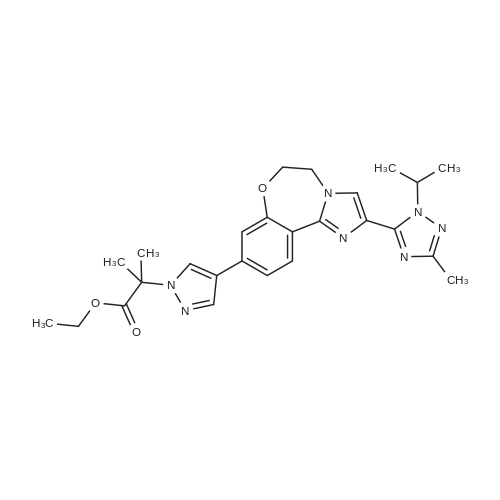
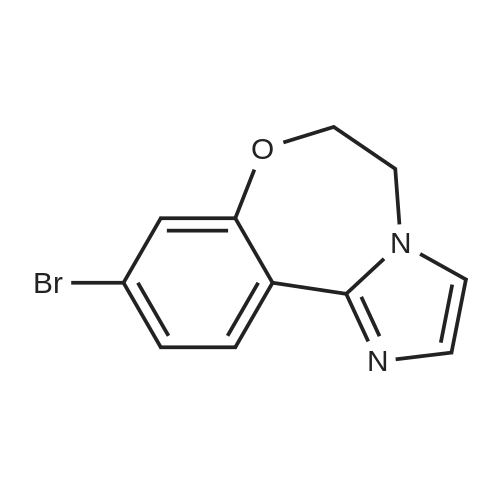
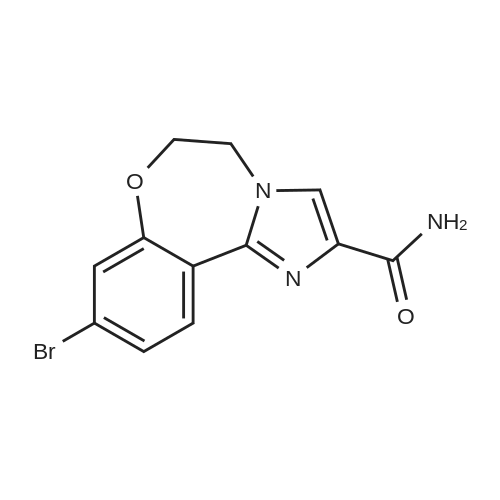
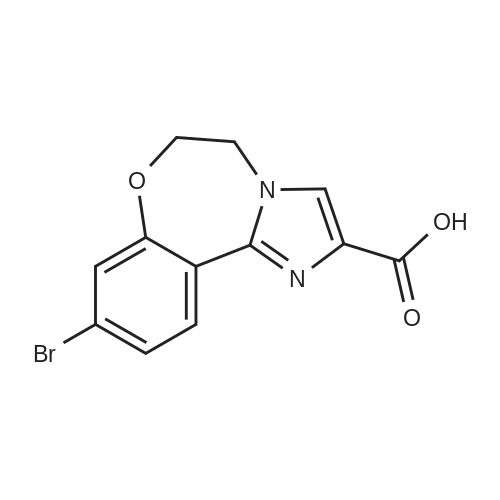
Step 3 2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)-1H-pyrazol-1-yl)-2-methylpropanoic acid 251 (90 mg, 0.2 mmol) was dissolved in DMF (2 mL) and treated with NH4Cl (40 mg, 0.8 mmol), DIPEA (0.3 mL, 2 mmol) followed by N,N,N',N'-tetramethyl-O-(7-azabenzotriazol-1-yl)uronium hexafluorophosphate (HATU, 100 mg, 0.4 mmol). The mixture was stirred at room temperature for 2 hours. Saturated sodium bicarbonate was added, and the mixture was extracted with EtOAc. The combined organics were dried over sodium sulfate and concentrated. The crude was purified by 10% MeOH/EtOAc following by trituration with minimal EtOAc to provide 74 mg (82% yield) of 196. LC/MS (ESI+): m/z 463(M+H). 1H NMR (500 MHz, DMSO) δ 8.44-8.26 (m, 2H), 8.01 (s, 1H), 7.86 (s, 1H), 7.44 (dd, J=8.4, 1.8, 1H), 7.35 (d, J=1.7, 1H), 7.15 (s, 1H), 6.79 (s, 1H), 5.82 (dt, J=13.3, 6.6, 1H), 4.52 (s, 4H), 2.25 (s, 3H), 1.75 (s, 6H), 1.47 (d, J=6.6, 6H) |
| 68% |
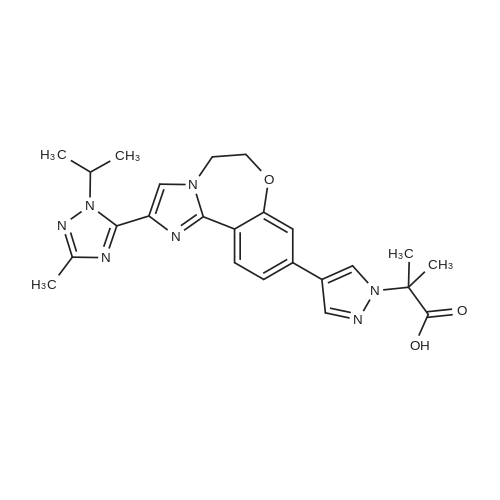
Stage #1: 2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)-1H-pyrazol-1-yl)-2-methylpropanoic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran at 22℃; for 1.08333h;
Stage #2: With ammonia In tetrahydrofuran; methanol at 45℃; |
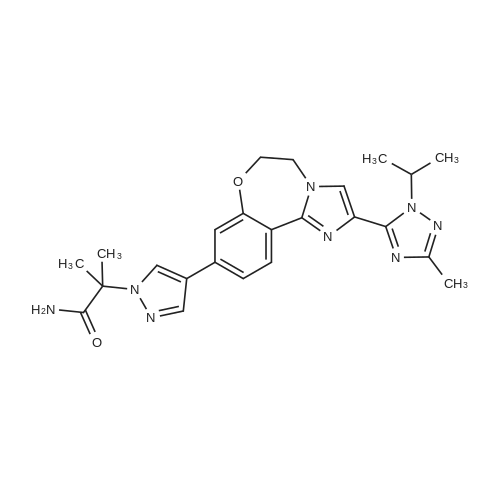
18 Example 18 2-(4-(2-( 1 -isopropyl-3-methyl- 1 H- 1 ,2,4-triazol-5-yl)-5 ,6- dihydrobenzo[f]imidazo[ 1 ,2-d] [ 1 ,4]oxazepin-9-yl)- lH-pyrazol- 1 -yl)-2-methylpropanamide I (GDC-0032)
2-(4-(2-(l-Isopropyl-3-methyl-lH-l,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[l,2- d][l,4]oxazepin-9-yl)-lH-pyrazol-l-yl)-2-methylpropanoic acid II was treated with di(lH- imidazol-l-yl)methanone (carbonyldiimidazole, CDI) in tetrahydrofuran followed by methanolic ammonia to give crude I. Solid II (1.44 kg, 3.12 mol, 1.00 equiv) was transferred into a 20 L bottle and then THF (10 L, 7 vol) was charged. The slurry was transferred under reduced pressure into a second 50 L reactor and additional THF (5 L, 3 vol) was added for the rinse. The internal temperature of the slurry was set to 22 °C and Γ1 -carbonyldiimidazole, CDI (0.76 Kg, 5.12 mol, 1.50 equiv) was charged to the mixture and a clear solution was observed after 5 min. The reaction mixture was sampled every 30 min and analyzed by HPLC (IPC: XTerra MS Boronic method) which showed almost complete conversion to the acyl-imidazole intermediate and 1.2% remaining II after 30 min. An additional portion of CDI (0.07 kg, 0.15 mol, 0.14 equiv) was added, and the reaction mixture was stirred for 1 h and then analyzed by HPLC (IPC: XTerra MS Boronic method) which showed 0.8% remaining II. Into a second 50-L reactor, was added NH3/MeOH (1.5 L, 10.5 mol, 3.37 equiv) and THF (5 L, 3 vol). The acyl-imidazole intermediate was transferred to a second reactor under reduced pressure (transfer time -10 min). The internal temperature was then set to 45 °C and the volume of solvent was distilled down from 35 L to 12 L. Water (6 L, 4 vol) was then added to the mixture that was further distilled from 18 L to 11 L. Finally, another portion of water (6 L, 4 vol) was added and the solvents were distilled one last time from 17 L to 14 L, until no more THF was coming out. The reaction was then cooled down to 10 °C (internal temperature). The white slurry was filtered and the filter cake was washed with water (2 x 6 L, 2 x 4 vol). The solids were then dried at 80 °C (jacket temp) in the Aurora filter for 24 h (KF = 1.5 % H20) under vacuum to give 1.25 kg crude I, GDC-0032 (84% corrected yield, 96% wt, 97.3 % pure by HPLC) as a white solid. A slurry of crude I (1.15 kg, 2.50 moles) in MeOH (6 L, 5 vol) was prepared and then charged to a 50 L glass reactor. Additional MeOH (24 L, 21 vol) was added to the mixture, which was then heated to 65 °C. A homogenous mixture was obtained. Si-thiol (Silicycle, Inc., 0.23 kg, 20% wt) was added to the solution via the addition port and the mixture was stirred for 3 hours. It was then filtered warm via the Aurora filter (jacket temperature = 60 °C, polish filtered and transferred directly into a second 50 L reactor with reduced pressure. The solution was then heated back to 65 °C internal temperature (IT). The homogeneous solution was cooled down to 54 °C and I seeds (12 g, 1 % wt) in MeOH (50 mL) were added with reduced pressure applied to the reactor. The mixture was then cooled down to 20 °C over 16 hours. The solids were then filtered via the Aurora filter and dried at 80 °C for 72 hours to give 921 g, 80% yield of I as a methanoate solvate (form A by XRPD,) and transferred to a pre-weighed charge-point bag. In an isolator, the solids were slurred in IP Ac (8 L, 7 vol) and transferred to a clean 10 L reactor. The mixture was stirred for 1 h at 60 °C (IT). The solids were then filtered via the Aurora system and dried at 80 °C (jacket) for 96 h. A sample of I was removed and analyzed by GC (IP Ac = 1 %). To attempt more efficient drying, the API was transferred to two glass trays in an isolator and sealed with a drying bag before being dried in a vacuum oven set at 100 °C for 16 h. GC (IPC: Q12690V2) showed 1 % solvent was still present. The process afforded 760 g (68% corrected yield, 68% wt, 99.9 % purity by LC) of a white solid (form B by XRPD). Crude I (340.7 g) was charged to a 2-1 . 1 1 DPI · bottle and slurried with 0.81 , isoamylalcohol (I A A). The slurry was transferred to a 20 L reactor and diluted with 6.7 L round- bottom flask (22 vol total). The white slurry was heated until a solution was observed (internal temperature rose to 118 °C and then cooled to 109 °C). The solution was polish filtered (0.2 μ .Μ filter). A flask was equipped with overhead stirring and the filtrate was slurried in isoamyl alcohol (344 ml ., 21 vol). The mixture was warmed to 95 °C (internal) until the solids dissolved. A slurry of charcoal (10 wt%, 0.16g) and silicycle thiol (10 wt%, 0.16g) in isoamyl alcohol (1 vol, 1 6 ml . ) was charged and the mixture was stirred at 90-95 °C for 1 h and then filtered (over Celite pad). The clear amber colored solution was cooled to 73 °C (seeding temp range = 70 ±5 °C) and a GDC-0032 I seed (10 wt%, 0.16g) was added. The temperature of the heating mantle was turned off and the mixture was allowed to cool to room temperature overnight with stirrin (200 rpni). After 17 hr, the white solids were filtered starting with slow gravity filtration and then vacuum was applied. The solids were suction dried for 20 min with mixing until a free flowing powder was obtained. ( "rude weight prior to oven drying = 16 g. The solids were oven- dried at 100 °C for 24 h and then sampled for testing. Drying continued at 100 °C for another 24 hr. I l l NMR (DMSO d6) δ 8.38 (t), 8.01 (s), 7.87 (s), 7.44, 7.46 (d), 7.36 (s), 7.18 (br s), 6.81 (br s), 5.82 (m), 3.99 (s), 2.50 (s), 2.26 (s), 1.75 (s), 1.48, 1.46 (d). Purified 2-(4-(2-(l-isopropyl-3-methyl-lH-l,2,4-triazol-5-yl)-5,6- dihydrobenzo[f]imidazo[ 1 ,2-d] [ 1 ,4]oxazepin-9-yl)- lH-pyrazol- 1 -yl)-2-methylpropanamide I (GDC-0032) was dry granulation formulated in tablet form by the roller compaction method (He et al (2007) Jour, of Pharm. Sci., 96(5):1342-1355) with excipients including lactose, microcrystalline cellulose (AVICEL PH 01, FMC BioPolymer, 50 μΜ particle), croscarmellose sodium (Ac-Di-Sol, FMC BioPolymer), and magnesium stearate |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping