|
|
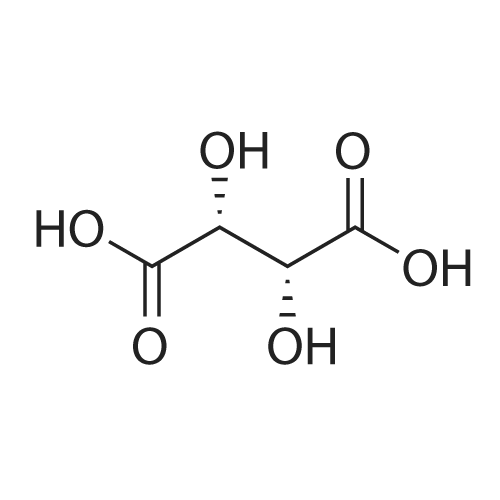
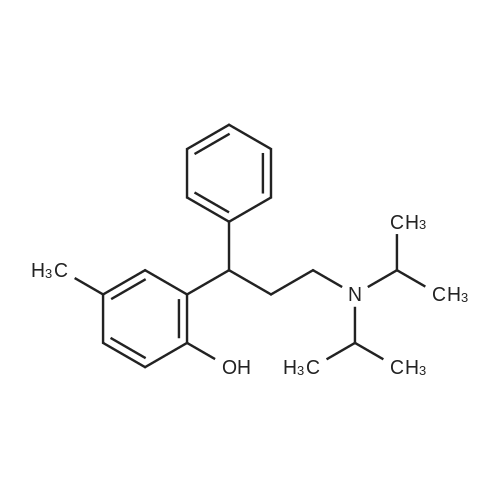
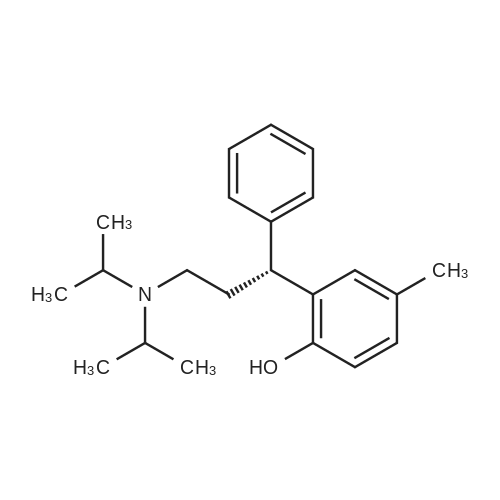
Example 4: Preparation of Tolterodine tartrate; Tolterodine hydrobromide (100 g, 0.246 mol), ethyl acetate (2 L) and water (500 ml) were mixed at room temperature in a glass reactor, forming a mixture. The mixture was stirred rapidly while adding potassium hydroxide (50%, 300 ml). After stirring thoroughly for approximately 15-30 minutes, two clear homogeneous layers formed. The layers were separated, and an organic phase was obtained. The organic phase was washed with water (2x with 500ml).L-tartaric acid (38.33 g) dissolved in ethanol (800 ml) was added rapidly in one portion to the organic phase, at room temperature, forming a slurry. The slurry was cooled to 00C +/-5C over 2 hours and maintained at this temperature for about 15 hours. The slurry was filtered using a suction filter, washed with cold ethanol (2x with 100 ml), and dried at about 60C under vacuum for about 10 hours, yielding Tolterodine tartarate (62.6 g). The Tolterodine tartrate was recrystallized from dry ethanol twice, yielding Tolterodine tartrate (41.2 g) of 99.84% purity as determined by HPLC. Level of impurities as determined by HPLC: RRT 0.18, 0.22, 0.50: 0% area, RRT 0.33: 0.01% area.; Example 5: Preparation of Tolterodine tartrate; Tolterodine hydrobromide (100 g, 0.246 mol), ethyl acetate (2 L) and water (500 ml) were mixed at room temperature in a glass reactor, forming a mixture. The mixture was stirred rapidly while adding potassium hydroxide (50%, 300 ml). After stirring thoroughly for approximately 30 minutes, two clear homogeneous layers formed. The layers were separated, and an organic phase was obtained. The organic phase was washed with water (2x with 500 ml). L-tartaric acid (38.4 g) dissolved in ethanol (800 ml) was added to the organic phase rapidly in one portion, at room temperature, forming a slurry. The slurry was cooled to 0C+/-5C over about 1 hour and maintained at this temperature for about 4 hours. The slurry was filtered using a suction filter, washed with cold ethanol (2x with 100 ml), and dried at about 60C under vacuum for about 10 hours to yield Tolterodine tartrate (65.2 g). The Tolterodine tartrate was recrystallized from dry ethanol, yielding Tolterodine tartrate (41.8 g) of 99.97% purity as determined by HPLC. Level of impurities, as determined by HPLC: RRT 0.18, 0.22, 0.33, 0.50: 0% area. EPO <DP n="18"/>; Example 6: Preparation of Tolterodine tartrate; Tolterodine hydrobromide (583 g, 1.434 mol), ethyl acetate (20 L) and water (5 L) were mixed at room temperature in a glass reactor, forming a mixture. The mixture was stirred rapidly while adding potassium hydroxide (50%, 1.5 L). After stirring thoroughly for approximately 30 minutes, two clear homogeneous layers formed. The layers were separated and an organic phase was obtained. The organic phase was washed with water (2x with 5 L).L-tartaric acid (385 g) dissolved in ethanol (8 L) was added rapidly in one portion to the organic phase, at room temperature, forming a slurry. The slurry was cooled to 0C +50C over about 1 hour and maintained at this temperature for about 12 hours. The slurry was filtered using a suction filter, washed with cold ethanol (2x with IL), and dried at about 6O0C under vacuum for 3 hours to yield Tolterodine tartrate (310 g). The Tolterodine tartrate was recrystallized twice from dry ethanol, yielding Tolterodine tartrate (219 g) of 99.98% purity as determined by HPLC. Level of impurities as determined by HPLC: RRT 0.18, 0.22, 0.33, 0.50: 0% area.; Example 7: Preparation of Tolterodine tartrate; Tolterodine hydrobromide (20 g, 0.049 mol), ethyl acetate (400 ml) and water (100 ml) were mixed at room temperature in a glass reactor, forming a mixture. The mixture was stirred rapidly while adding potassium hydroxide (50%, 35 ml). After stirring thoroughly for approximately 30 minutes, two clear homogeneous layers formed. The layers were separated and an organic phase was obtained. The organic phase was washed with water (2x with 100 ml).The organic phase was added to L-tartaric acid (7.7 g) dissolved in ethanol (160 ml) over about 30 minutes at room temperature, creating a slurry. The slurry was cooled to 00C +/-5C over about 2 hours and maintained at this temperature for about 4 hours. The slurry was filtered using a suction filter, washed with cold ethanol (2x with 20 ml), and dried at about 600C under vacuum for about 14 hours to yield Tolterodine tartrate (12.5 g).The Tolterodine tartrate (8.5 g) was recrystallized twice from dry ethanol, yielding Tolterodine tartrate (6.0 g) of 99.98% purity as determined by HPLC. Level of impurities as determined by HPLC: RRT 0.18, 0.22, 0.33, 0.50: 0% area. EPO <DP n="19"/>; Example 8: Preparation of Tolterodine tartrate; Tolterodine hydrobromide (20 g, 0.049 mol), ethyl acetate (400ml) and water (100 ml) were mixed at room temperature in a glass reactor, forming a mixture. The mixture was stirred rapidly while adding potassium hydroxide (50%, 35 ml). After stirring thoroughly for approx... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping