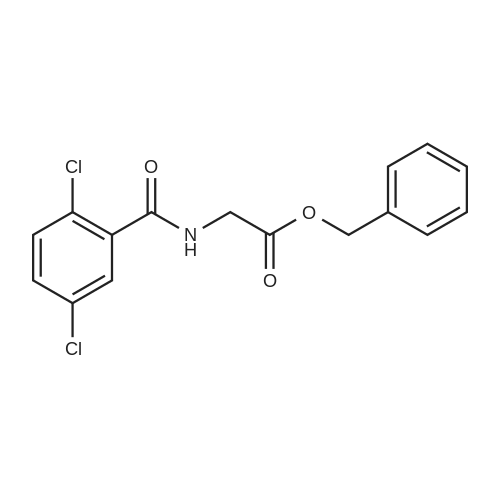
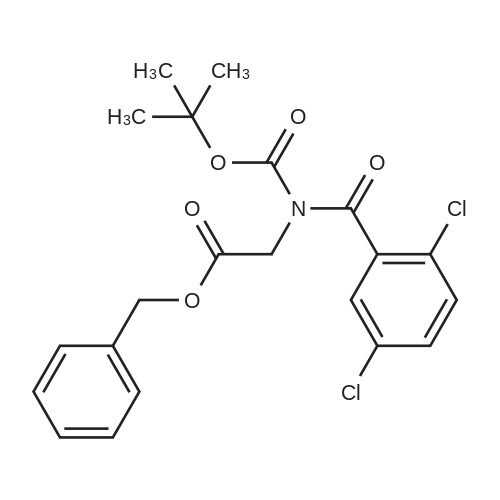
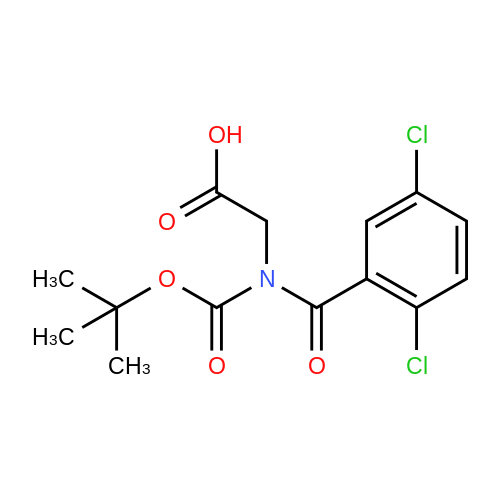
| 91% |
With boric acid In acetone at 20℃; for 22h; |
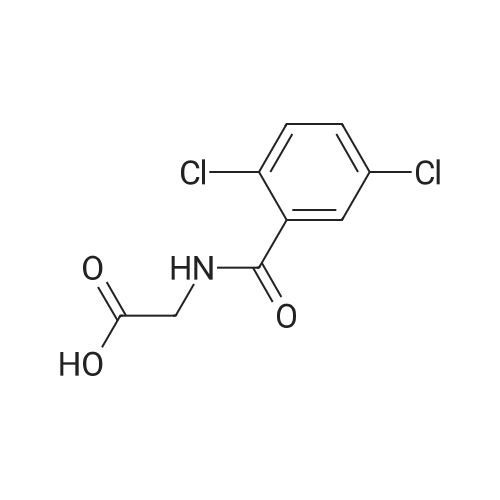
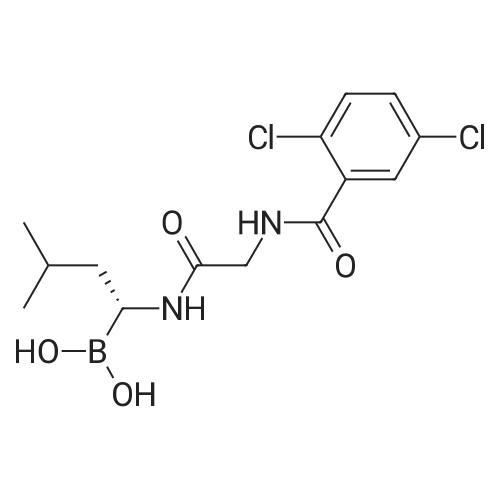
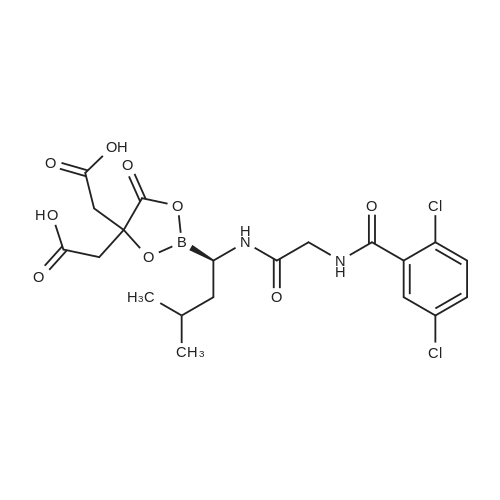
3 Synthesis of ixazomib citrate
3.00 g (6.06 mmol) of 2,5-dichloro-N-[2-({( R)-3-methyl-1-[(3aS,4S,6S,7aR)-3a,5,5- trimethyihexahydro-4,6-methano-1 ,3,2-benzodioxaborol-2-yl]butyl}amino)-2- oxoethy]benzamide of formula V, 0.41 g (6.67 mmol) of boric acid and 1.28 g (6.67 mmo) of citric acid are weighed into a flask. The mixture is stirred in 15 ml of acetone at the room temperature for 22 h. 50 ml of ethyl acetate is added to the mixture. After removal of a part of the solvent (15 ml) by distillation and spontaneous cooling of the reaction mixture, Ixazomib citrate of formula I is obtained by aspiration. After drying in a vacuum drier (16 h at 40°C) the yield of the reaction is 91 % and HPLC purity of the product is 98.9%. 1H NMR (DMSO, 500.13 MHz, 25°C): 12.15 (br.s., 2H), 10.71 (br.s., 1 H), 9.13 (t, 1 H, J - 5.7 Hz), 7.66 (s, 1H), 7.57 (d, 2H, J = 1.4 Hz), 4.27 (br, 2H), 2.92 - 2.87 (m, 1H), 2.77 - 2.73 (m, 1 H), 2.66 - 2.59 (m, 1H), 2.53 (br, 2H), 1.69 (br, 1H), 1.38 - 1.18 (m, 2H), 0.88 (d, 6H, J = 6.4 Hz); 1H NMR (DMSO, 250.13 MHz, 80 °C): 10.29 (br.s., 1H), 8.82 (t, 1 H, J = 5.7 Hz), 7.62 (t, 1H, J = 1.5 Hz), 7.53 (d, 2H, J = 1.5 Hz), 4.27 (d, 2H, J - 5.6 Hz), 2.76 - 2.62 (m, 5H), 1.79 - 1.63 (m, 1H), 1.44 - 1.20 (m, 2H), 0.897 (d, 3H, J = 6.5 Hz), 0.892 (d, 3H, J - 6.4 Hz); The XRPD pattern is shown in Fig. , the characteristics peaks are presented in Table 1. We refer to this novel form of Ixazomib citrate as Form 3; The DSC record contains an endotherm that corresponds to the melting point Tonset= 22.2°C. The record is shown in Fig. 2 in the Appendix; The TGA record indicates a weight loss in the temperature range of 20 to 200°C of about 1.3%. The record is shown in Fig. 3 in the Appendix. |
| 84% |
In ethyl acetate at 50 - 70℃; for 24h; |
2.2 Step 2:
Citric acid (6.4 g, 33 mM, 1.2 eq)Was dissolved in 70 ° C ethyl acetate (400 mL)Then add the product on the step,After stirring at 50 ° C for 24 hours,Cooled to room temperature, filtered, washed with ethyl acetate,Dried to give the desired product 11.9g, yield 84%Its purity was 97.4% by HPLC. |
| 1908 g |
With hydrogenchloride In water; acetone at 45 - 50℃; for 4.5h; Large scale; |
6 Example 6. Preparation of form 2 of Ixazomib Citrate having high levels of residual solvents
A double jacket glass reactor was charged with 2,5-dichloro-N-[2- [[(1 R)- 1 -[(3aS,4S,6S,7aR)-hexahydro-3 a,5,5-trimethyl-4,6-methano-1 ,3,2-benzodioxaborol-2-ylj-3-methylbutylj aminoj-2-oxoethylj benzamide (2000 g), citric acid monohydrate (1103 g), conc. aqueous HC1 (51 mL) and acetone (20 L). Obtained solution was warmed to about 45 °C and stirred at 45 - 50 °C for about 4.5 hours. The solution was concentrated under reduced pressure (about 30 kPa, process temperature 25 - 40 °C, internal temperature about 55 °C) to about one third of the original volume (about 7 L) and the residue was diluted with ethyl acetate (10 L). Obtained mixture was concentrated to about 10 L and diluted again with ethyl acetate (10 L). Obtained mixture was concentrated to about 16 L and diluted again with ethyl acetate (14.8 L). Obtained mixture was concentrated to about 23 L and then it was warmed to 45 -50 °C. After about 2 hours treatment at 45 - 50 °C, the mixture was cooled to about 25 °C during about 1.5 hour and stirred at about 25 °C for additional 1 hour. Insoluble solid was separated by filtration, washed with ethyl acetate (3 x 5 L) and dried in vacuum oven at temperature about 50 °C and pressure below 100 mbars to give Ixazomib citrate Form 2 (1908 g, 727 ppm of acetone, 1 % of ethyl acetate), as identified by XRPD. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping