|
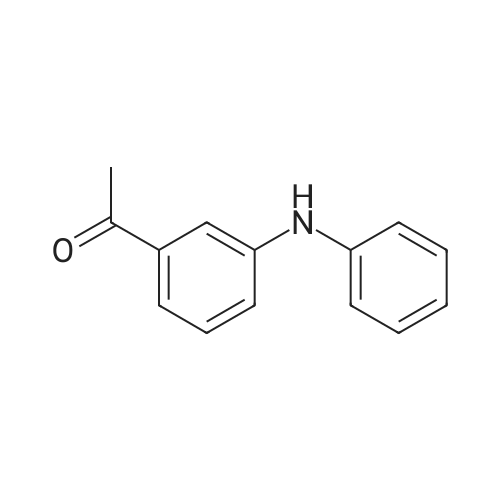
In <i>N</i>-methyl-acetamide; dichloromethane; water; ethyl acetate |
32.6 Step 6
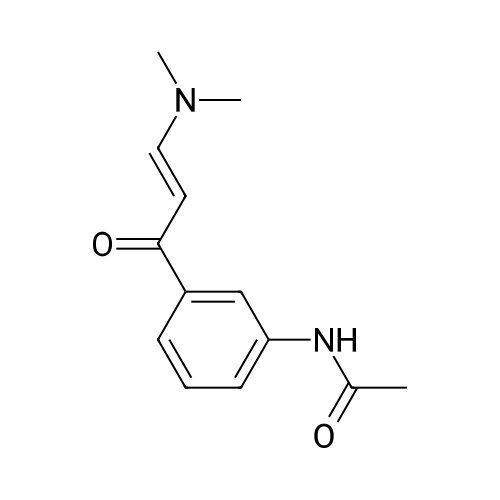
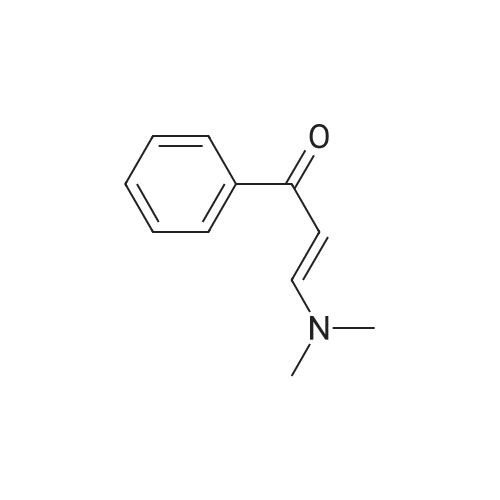
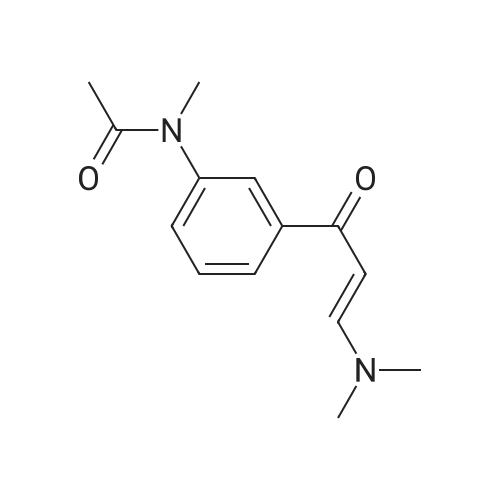
Step 6 N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]-N-methylacetamide N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]acetamide (3.77 kg) is suspended in dimethylformamide (20 L; Van Waters) and the mixture chilled in an ice bath. Sodium hydride (808 g, 60% dispersion; Aldrich) is added to the suspension under a nitrogen atmosphere. The temperature of the reaction mixture is maintained below 10?C. during the addition of the hydride. After the addition is complete the mixture is stirred for 1 hour, then methyl iodide (2.46 kg) is added slowly while maintaining the temperature below 10?C. The reaction mixture is stirred overnight and allowed to come to room temperature. HPLC analysis of the reaction mixture shows 97.7% product and ~2.3% of the starting material. The addition of methyl iodide (53 g; Aldrich) and continued stirring (5 hours) does not change this ratio. The reaction mixture is quenched by the addition of 1 L of water. The mixture is triturated with hexanes (2*4 L) which are discarded. Most of the DMF is removed under reduced pressure. The residue is diluted with water (6 L) and product is extracted with methylene chloride (20 L; Barton). The solution is dried over sodium sulfate, filtered and the solvent evaporated to give a solid. This material is triturated with hexanes (15 L) and ethyl acetate (15 L). The slurry was cooled to room temperature, filtered and washed with hexanes (0.5 L). This material is found to be only ~91% product by HPLC (area %). The material is purified by column chromatography. The material is dissolved in methylene chloride and passed through a pad of silica (~18 kg). The polarity of the eluant is gradually increased by adding ethyl acetate (Barton). Eventually the column is flushed with ethyl acetate. In this manner 2.4 kg of N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]-N-methylacetamide is obtained with a purity of 98.05% by HPLC (area %). |
|
In <i>N</i>-methyl-acetamide |
137 N-[3-[4-(Chloroacetyl)-(3-cyano-4.5-dihydropyrazolo[1,5-a]pyrimidinyl)phenyl]-N-methylacetamide
EXAMPLE 137 N-[3-[4-(Chloroacetyl)-(3-cyano-4.5-dihydropyrazolo[1,5-a]pyrimidinyl)phenyl]-N-methylacetamide To 33.0 g of 3'-acetamido-3-dimethylaminoacrylophenone in 170 ml of dimethylformamide was added 6.8 g of 60% sodium hydride followed by 40 g of methyl iodide to give, after workup, 10.5 g of 3'-(N-methylacetamido)-3-(N,N-dimethylamino)acrylophenone, m.p. 129°-131° C. |
|
In <i>N</i>-methyl-acetamide; hexane; dichloromethane |
173 N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]-N-methylacetamide
EXAMPLE 173 N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]-N-methylacetamide A solution of 4.62 g of N-[3-[3-(dimethylamino)-1-oxo-2-propenyl]phenyl]acetamide in 25 ml of dimethylformamide was stirred in an inert atmosphere and 1.0 g of sodium hydride (60% oil suspension) was added. After stirring for 1 hour, the liberation of hydrogen had ceased and a solution of 3.0 g of methyl iodide in 10 ml of dimethylformamide was gradually added (with cooling, if necessary). After stirring for an additional 1 hour at room temperature, any volatiles were removed at reduced pressure and then the reaction mixture was triturated 3 times with 100 ml of hexane. The reaction mixture was carefully poured into cold water and extracted exhaustively with methylene chloride. This material was evaporated at reduced pressure to yield a yellow-orange solid. A solution of the crude solid in methylene chloride was passed through a pad of hydrous magnesium silicate. Addition of hexane to the refluxing elude gave crystals which were collected after cooling. The desired compound was a yellow-orange crystalline material, mp 146°-148° C. |
|
In <i>N</i>-methyl-acetamide; dichloromethane; water; ethyl acetate |
32.6 Step 6:
Step 6: N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]-N-methylacetamide N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]acetamide (3.77 kg) is suspended in dimethylformamide (20 L; Van Waters) and the mixture chilled in an ice bath. Sodium hydride (808 g, 60% dispersion; Aldrich) is added to the suspension under a nitrogen atmosphere. The temperature of the reaction mixture is maintained below 10?C during the addition of the hydride. After the addition is complete the mixture is stirred for I hour, then methyl iodide (2.46 kg) is added slowly while maintaining the temperature below 10?C. The reaction mixture is stirred overnight and allowed to come to room temperature. HPLC analysis of the reaction mixture shows 97.7% product and ~2.3% of the starting material. The addition of methyl iodide (53 g; Aldrich) and continued stirring (5 hours) does not change this ratio. The reaction mixture is quenched by the addition of 1 L of water. The mixture is triturated with hexanes (2 X 4 L) which are discarded. Most of the DMF is removed under reduced pressure. The residue is diluted with water (6 L) and product is extracted with methylene chloride (20 L; Barton). The solution is dried over sodium sulfate, filtered and the solvent evaporated to give a solid. This material is triturated with hexanes (15 L) and ethyl acetate (15 L). The slurry was cooled to room temperature, filtered and washed with hexanes (0.5 L). This material is found to be only ~91% product by HPLC (area %). The material is purified by column chromatography. The material is dissolved in methylene chloride and passed through a pad of silica (~18 kg). The polarity of the eluant is gradually increased by adding ethyl acetate (Barton). Eventually the column is flushed with ethyl acetate. In this manner 2.4 kg of N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]-N-methylacetamide is obtained with a purity of 98.05% by HPLC (area %). |
|
With potassium hydroxide In <i>N</i>-methyl-acetamide; dichloromethane; water |
2 Preparation of N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]-phenyl]-n-methylacetamide (Formula III)
Example 2 Preparation of N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]-phenyl]-n-methylacetamide (Formula III) To a suspension of N-[3-[3-(dimethylamino)-1-oxo-2-propenyl]-phenyl]-acetamide (230 gm) in dimethylformamide (1150 ml) was added powdered potassium hydroxide (110.90 gm) and the reaction mixture was stirred at about 25° C.-30° C. for about 30 min. The reaction mass was cooled to about 0° C.-5° C. and methyl iodide (154.67 gm) was added in about 30 min at about the same temperature. After complete addition, the temperature was allowed to rise to about 20° C.-25° C., and while stirring, the reaction mass at about the same temperature until the reaction is completed (~3 hrs) as monitored via thin-layer chromatography (TLC). To the reaction mass was added methylene chloride (500 ml) and water (500 ml), separated the organic layer and the aqueous layer was extracted with 2*500 ml methylene chloride. The combined organic layers were washed with 3*500 ml water, the subsequent organic layer was dried over sodium sulfate and concentrated to dryness to give a yellow colored solid, which was triturated with n-hexane (700 ml), filtered and dried at about 50° C. until a constant weight to give a solid product (190 gm) (Purity, ~>95%, by TLC). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping