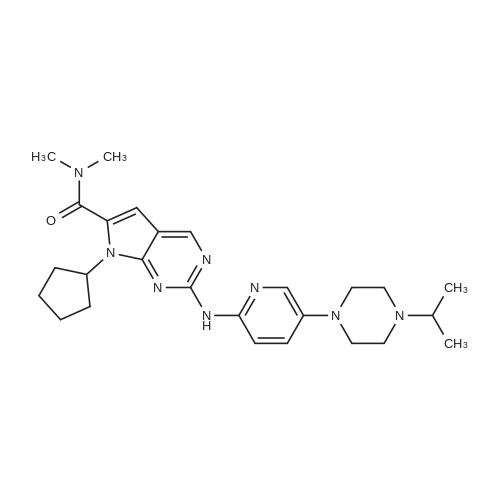
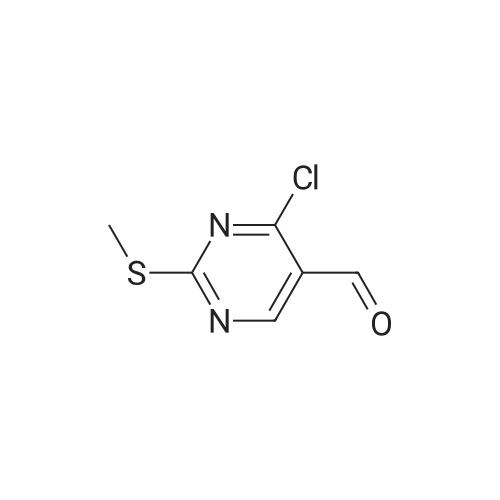
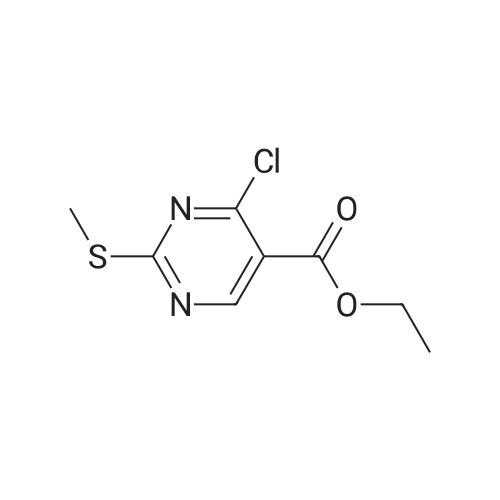
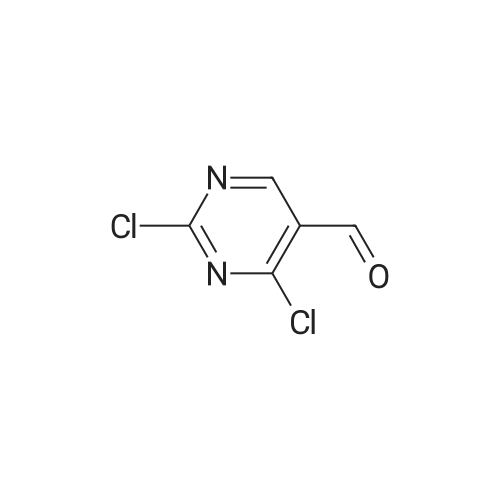
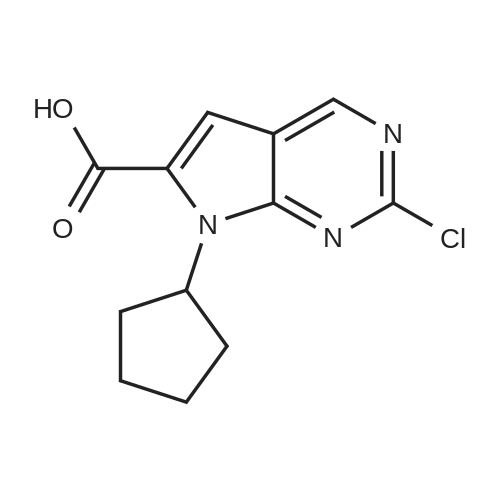
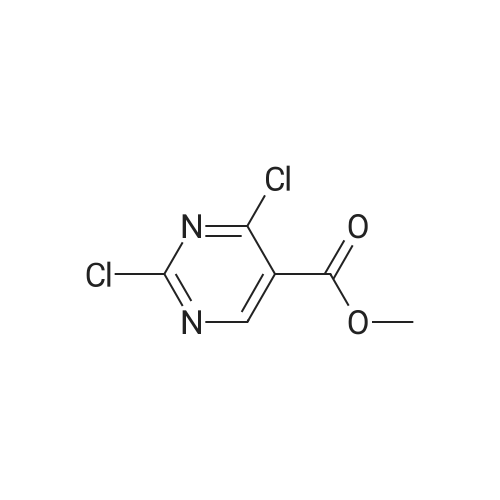
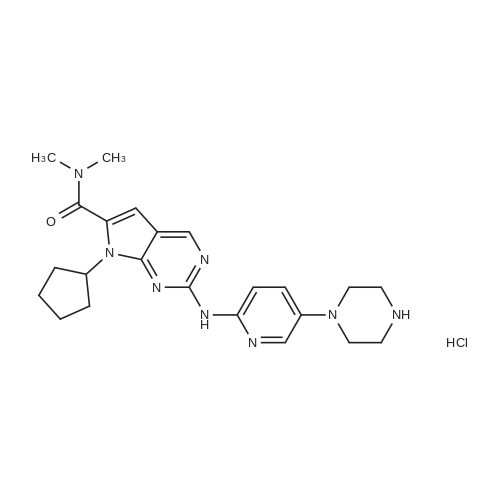
| 91.1% |
With hydrogenchloride; In water; at 20 - 30℃; |
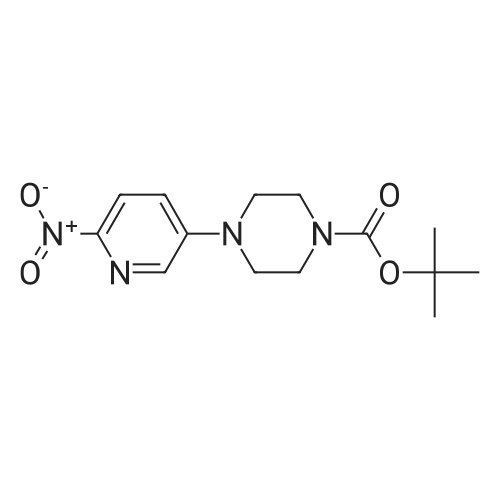
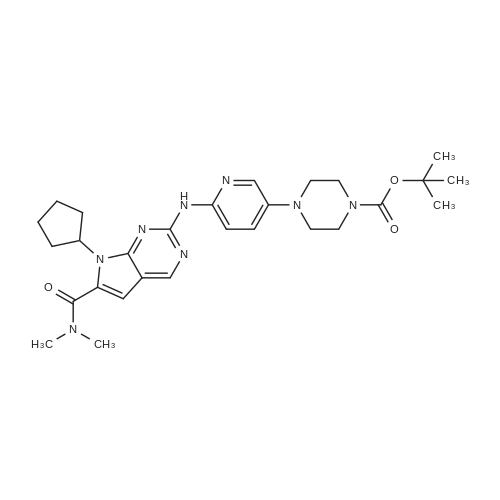
Tert-butyl 4-(6- { [7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrolo[2, 3-d]pyrimidin-2-yl]amino } pyridin-3 -yl)piperazine- 1 -carboxylate (100g) was added to the solution of distilled water (200 ml) and conc. HC1 (250 ml) and stirred at 20-30C. The reaction mass was slowly added into pre-chilled aq. NaOH at 0-10C. Isopropyl alcohol (200 ml) and dichloromethane (500 ml) was added to the reaction mixture. The organic layer was concentrated under vacuum and to theresidue was added dichloromethane (100ml) and heptane (500ml). The mixture was stirred at 20-30C. The solid was filtered and dried under vacuum at 60-70C and collected as off-white to pale yellow solid.Yield: 91.1.0 %; HPLC Purity: 99.89%?H NMR (400 MHz DMSO) ppm 9.34-9.3 1 (br,1H), 8.77 (s, 1H), 8.18-8.15 (d,J=9.2, 1H), 8.03-8.02 (d, J=2.8, 1H), 7.46-7.43(dd, J=2.8, J=9.2 , 1H), 6.59 ( s,1H), 4.77- 4.69 (m, 1H), 3.05-3.01 (m, 10 H), 2.84 (m, 4H), 2.43 (m, 2H), 2.19 (m, 1H), 1.97 (m, 4H), 1.64-1.63 (m, 2H) |
| 91% |
With hydrogenchloride; water; at 25 - 45℃; |
DM water (200.0 mL) and tert-butyl 4-[6-[[7-cyclopentyl-6-(dimethyl carbamoyl)pyrrolo[2,3-d]pyrimidin-2-yl]amino]-3-pyridyl]piperazine-l-carboxylate (40.0g, 0.075 moles) were charged into 1L 4N RB flask at 30±5C and stirred for 5- (0137) 10 min. to get cream colored suspension. Reaction mass was heated to 40-45C and diluted aq.hydrochloric acid (199.0 mL, 0.561 moles, prepared by diluting 49.0 mL of Cone hydrochloric acid with 150 mL of DM water) was added dropwise to the above reaction mass and stirred for about lh to get pale brown colored clear solution. Resulting clear solution was further stirred for l-2h for reaction completion. (0138) After reaction completion (by HPLC), reaction mass was diluted with DM water (80 mL) and stirred for 5-l0min. Reaction mass was washed with ethyl acetate (200 mL x 2) and layers were separated. Aqueous layer was basified using aq.sodium hydroxide solution (45. Og of sodium hydroxide was dissolved in 900.0 mL of DM water) and stirred the resulting cream colored suspension for lh at 25-35 C. Product was filtered under suction with the help of DM water (200 mL). Wet product was leached from DM water (400 mL) followed by methanol (200 mL) and product was filtered and dried in hot air oven for 6h at 70-75C. Weight of the product: 29.6g (91.0% by theory). Purity by HPLC > 99.0%. |
| 90% |
With hydrogenchloride; In methanol; water; at 10 - 25℃; |
tert-butyl 4-(6-((7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl)amino)pyridin-3-yl)piperazine-1-carboxylate (53.47g, 100mmol) and methanol (267mL) were added to a three-neck flask. stirred uniformly, cooled to 10 ~ 15 C, added dilute hydrochloric acid (4mol / L, 267mL), after heating, The reaction is carried out at 20 to 25 C for 3 to 4 hours. At the end of the reaction, a part of methanol was rotated, and methyl t-butyl ether (267 mL) was added for At the end of the reaction, a part of methanol was rotated, and methyl t-butyl ether (267 mL) was added for extraction once.The collected aqueous phase was cooled to 0-10 C, dichloromethane (267 mL) was added, 20% sodium hydroxide solution was added dropwise to adjust the pH to 12-13, and the aqueous phase was extracted twice with dichloromethane (133 mL). The organic phase was washed twice with saturated brine (267 mL), concentrated, and then isopropyl alcohol and water were beaten.The solid was isolated by filtration and dried in vacuo to give the product ribociclib (39.11 g, 90%). |
| 85% |
|
In a 1 L four-necked flask was added 20 g of 4-(6-(7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl)aminopyridine- 3-yl) piperazine-1-carboxylic acid tert-butyl ester) (formula 3), 100g of n-hexane, stirred and dissolved, dripped at room temperature100 g of 3 mol/L hydrochloric acid was added, and the reaction was carried out for 1 hour, and the liquid layer was separated, and 200 g of acetone was added to the aqueous layer. Warming up to reflux, then loweringThe crystals were warmed to below 10 C, stirred for 3 hours, and filtered to obtain Rebsini hydrochloride.Example 3 Preparation of Rebizzini (Formula 4)Dissolve Rebsini hydrochloride in 200g water, add 1g activated carbon, 0.5g 100~200 mesh column chromatography silica gel, stirDecolorization by adsorption, filtration, adding saturated sodium bicarbonate solution to the filtrate to adjust the pH to 9-10, precipitate solids, cool down to 10Stir at C. After filtration, the cake was dried at 60 C to obtain 14 g of Rebizzini (formula 4) in a yield of 85.0%. |
| 83.7% |
With hydrogenchloride; In water; toluene; at 20℃; for 2h; |
In a 250 ml round bottom flask,Was added 2-chloro-7-cyclopentyl--N, N- dimethyl--7H- pyrrolo [2,3-d] pyrimidine-6-carboxamide and 14.6g (50mmol),Tert-Butyl 4- (6-aminopyridin-3-yl) piperazine-1-carboxylate15.3 g (55 mmol),0.67 g (3 mmol) of Pd (OAc) 2,BINAP 1.2 g (2 mmol),Cs2CO3 65.1g (200mmol) and 130ml 1,4-dioxane, warmed to 90 C, the reaction was stirred for 8 hours,The reaction was monitored, concentrated under reduced pressure and flash column chromatographyA solution of 4- (6- (7- (dimethylcarbamoyl) -7H-pyrrolo [2,3-d] pyrimidin- 2-ylaminopyridin-3-yl) - carboxylic acid tert-butyl ester.The above obtained solution of 4- (6- (7- (dimethylcarbamoyl) -7H-pyrrolo [2,3-d] pyrimidin- 2-ylaminopyridin-3-yl) - carboxylic acid tert-butyl esterDissolved in 100ml of toluene,Add 30ml 6mol / L hydrochloric acid, and stirred at room temperature for 2 hoursThe reaction was monitored completely, adjusted to pH 8 with sodium hydroxide, extracted with methylene chloride, washed with brine and driedThe organic phase was dried over sodium sulfate, concentrated under reduced pressure,N-hexane to obtain Ribociclib 18.2g, the yield was 83.7% |
| 81% |
With hydrogenchloride; In ethanol; water; at 20℃; for 4h; |
4- [6-[(7-cyclopentyl) -6-[(dimethylamino) carbonyl] -7H-pyrrolo [2,3] pyrimidin-2-yl] amino]- 3-pyridyl] -1-piperazinecarboxylic acid 1,1-dimethylethyl ester (10.7 g, 0.02 mol) was dissolved in 200 ml of ethanol,10 ml of 3N hydrochloric acid was added, and the reaction was stirred at room temperature for 4 h.After the reaction, 10% sodium hydroxide aqueous solution was added dropwise to adjust the pH of the reaction solution to 8-9, ethanol was distilled off under reduced pressure, and a pale yellow solid was obtained by suction filtration.Acetone was recrystallized to give 7.0 g of a white solid, yield: 81%. |
| 66.1% |
With hydrogenchloride; water; In acetone; at 50℃; for 2.16667h;Inert atmosphere; |
Under inert atmosphere, tert-butyl 4-(6-(6-(dimethylcarbamoyl)-7-cyclopentyl-7H- pyrrolo[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)piperazine-l-carboxylate (40.0 g) was suspended in 3 : 1 water/acetone mixture (800 mL) and the suspension was heated to 50 C. Aq. hydrochloric acid 6 M (60.8 mL) was added dropwise in 10 min and the mixture was kept under stirring at 50 C for 2 h. At the end of reaction, the solution was cooled down to 25 C and filtrated on a dicalite pad. Aq. 10%> sodium hydroxide (136 mL) was added dropwise in 10 min and the suspension was kept under stirring for 30 min. Ribociclib base was isolated by filtration, washed with water (120 mL) and dried under vacuum at 20 C for 16 h. Ribociclib was obtained as a solid (66.1% yield). |
|
|
A nitrogen-flushed, 3 L Argonaut reactor system is charged with 67.4 g (0.126 mol, 1.0 eq.) of tert-Butyl 4-(6-(7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrolo[2,3-d]pyrimidin-2-ylaminopyridin-3-yl)piperazine-1-carboxylate (A3) and 329 g (380 mL) of toluene. The suspension is stirred and cooled to 12±3 C. 138 g (126 mL, 6.0 eq) of 6N aqueous hydrochloric acid is added over 30 min maintaining a batch temperature 15±3 C. The resulting 2-phase solution is warmed to 25±3 C. and held at this temperature for 30 min or until the remaining starting material, A3, is 2% (area normalization) as determined by HPLC analysis. The progress of the reaction is checked using the Process Steering Control. 250 g (250 mL) of 1N aqueous hydrochloric acid is added and mixture is stirred for 5 min. The 2-phase reaction mixture is filtered through 25 g of filter cel. The phases are separated. The aqueous phase (containing product) is charged to a 2 L, 4-neck, round bottom flask (equipped as described under Apparatus entry 4) and cooled to 15±3 C. The pH is adjusted to 3.2±0.3 with the slow addition of 62 g (41 mL) of 50% aqueous sodium hydroxide, a batch temperature?27±3 C. is maintained throughout the addition. 16.4 g of Si-Thiol functionalized silica gel is added. The slurry is stirred for 3 hours at 50±3 C. The resin is filtered off, the flask and filter cake are rinsed with 50 mL of water. The wash is combined with the filtrate. The filtrate is transferred back to the flask and 16.4 g of Si-Thiol functionalized silica gel is added. The slurry is stirred for 3 hours at 50±3 C. The silica gel is filtered off. The flask and filter cake are rinsed with 50 mL of water. The wash is combined with the filtrate. The filtrate is transferred back to the flask and again 16.4 g of Si-Thiol functionalized silica gel is added. The slurry is stirred for 3 hours at 50±3 C. The silica gel is filtered off. The flask and filter cake are rinsed with 50 mL of water. The wash is combined with the filtrate. A nitrogen-flushed, 3 L, Argonaut reactor system is charged with the filtrate and cooled to 15±3 C. The pH is adjusted to 12.5±0.5 with the slow addition of 17 g (18 mL) of 50% aqueous sodium hydroxide to precipitate the product (Batch volume=900 mL, Max Vol). The sample is stirred for at least 6 h at 22±3 C. The solids are filtered through a polypropylene filter pad. The filter cake is washed four times with 340 g (4*85 mL) of water until the pH of the wash is ?9. The solids are dried at 60 C. for at least 16 h or until the LOD is ?1% to afford 45.7 g (84.9%, corrected) of compound A4 as a tan solid, mp 194-195 C. |
|
With hydrogenchloride; In water; ethyl acetate; for 10h; |
(11) 300 mg of the compound of formula (13) was dissolved10 ml of ethyl acetate,Add 5ml concentrated hydrochloric acid,Reaction for 10 hours,After the reaction is complete,Adding the extractant and water extraction to remove the impurities dissolved in the organic phase,Add sodium bicarbonate to the water phase to alkaline,place,Precipitate pure product7-cyclopentyl--N, N- dimethyl-2 - ((5- (piperazin-1-yl)Pyridin-2-yl) amino) -7H-pyrrolo [2,3-d]Pyrimidine-6-carboxamide(Compound of formula (a)) |
| 35.1 g |
With hydrogenchloride; In water; toluene; at 10 - 30℃; for 1.16667h; |
A solution of tert-butyl 4-(6-((7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrolo[2,3- d]pyrimidin-2-yl)amino)pyridin-3-yl)piperazine-l-carboxylate (Ila) (50 g) in toluene (300 mL) was cooled to 10 C. Hydrochloric acid (6N, 100 mL) was added to the above solution in drop wise at 15 C. The reaction mass was stirred for 1 hour at 25-30 C. Hydrochloric acid (IN, 200 mL) was added to the above reaction mass at 25-30 C and stirred for 10 minutes at the same temperature. The reaction mass was then filtered and washed with toluene (1 x 50 mL) followed by hydrochloric acid (IN, 50 mL). Organic layer was separated and aqueous layer was cooled to 15 C. pH of aqueous layer was adjusted to pH=12.0-12.5 using saturated solution of sodium hydroxide (50% w/w) and stirred for 1 hour at 15-20 C. The resulted precipitate was then filtered, washed with water (3 chi 80 mL) and dried at 50 C for 6 hours to provide the title compound. (0169) Yield: 35.1 g; Purity by HPLC: 99.31 % |
|
With hydrogenchloride; In water; at 8℃; |
In a nitrogen environment at about 25±2 C., mix 2-chloro-7-cyclopentyl-N,N-dimethyl-7H-pyrrolo[2,3-d]pyrimidine-6-carbamide and 4-(6-aminopyyrol-3-yl)piperazin-1-carboxyl tert-butylate into tetrahydrofuran THF) added with lithium bis(trimethyl)amine (LiHMDS) and stir for about 1 h to obtain the intermediate 4-[6-[[7-cyclopentyl-6-[(dimethylamino)carbonyl]-7H-pyrrolo[2,3-pyrimidine-2-yl]amino]-3-pyridine]-1-piperazinecarboxyl 1,1-dimethylethylate. Cool the mixture to about 8±2 C. and keep the mixture at this temperature while an aqueous hydrogen chloride solution is subsequently added slowly and mixed into the mixture. After that, using a separatory funnel and ethyl acetate as an extracting agent, perform an extraction process in duplicate to acquire the aqueous phase. When the extracted solution is cooled to about 5 C. or lower, slowly add an aqueous sodium hydroxide solution until the pH reaches 12.5. Heat the solution to 25 C. and stir for about 16 h. Next, filter the solution to obtain the solid matter (or filter cake), and rinse the filter cake with DD water until the pH of the rinsing liquid is equal to or lower than 9. Lastly, dry the filter cake at about 55±5 C. to yield yellowish brown solids, which are 7-cyclopentyl-N,N-dimethyl-2-[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide, whose molar recovery rate can be 98% or higher, with 98% or higher purity. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping