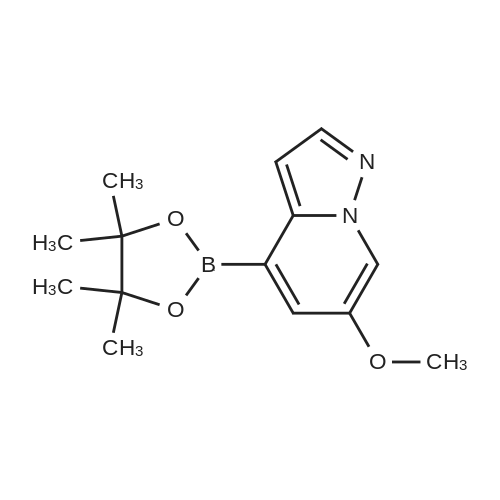
|
|
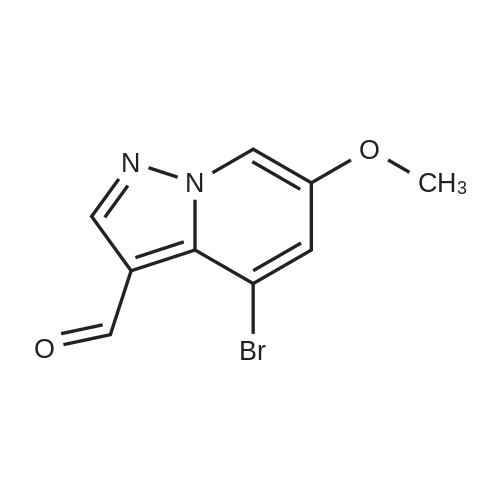
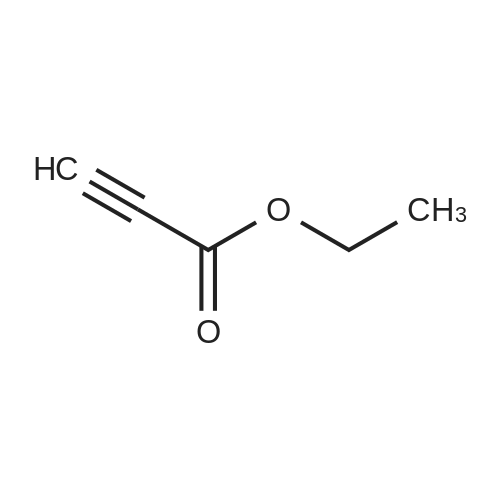
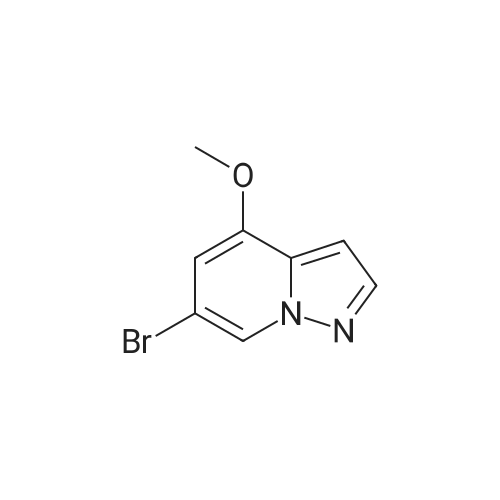
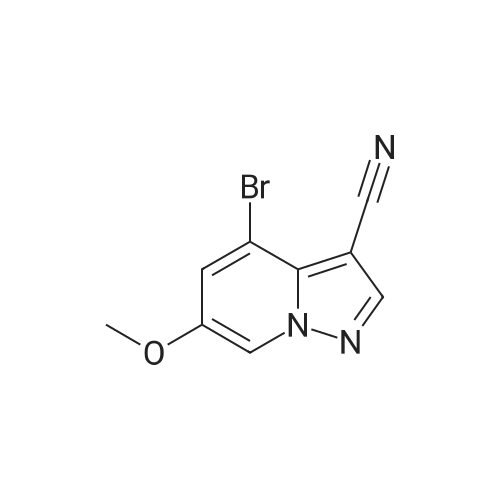
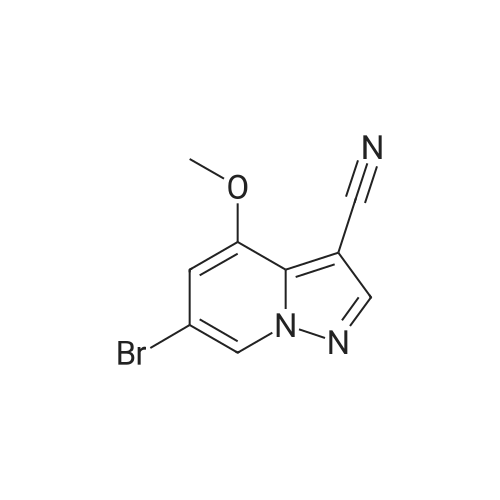
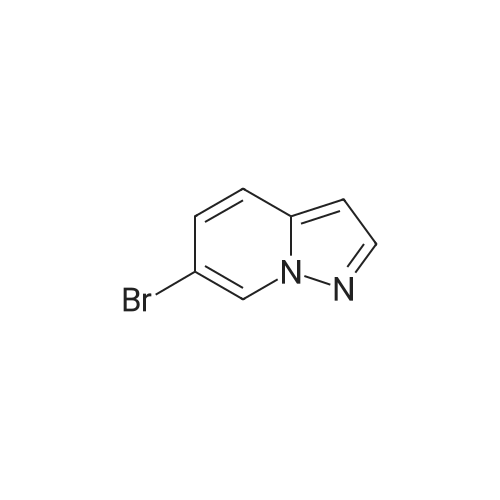
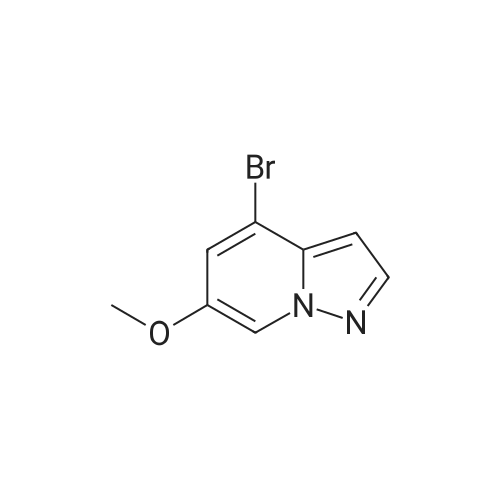
To a magnetically stirred white suspension of 1-amino-3-bromo-5-methoxypyridin-1-ium-2,4,6-trimethylbenzenesulfonate (33.24 g, 82.42 mmol) in DMF (82 mL) at ambient temperature was added TEA (22.98 mL, 164.8 mmol), followed by dropwise addition of ethyl propiolate (16.71 mL, 164.8 mmol). After vigorous stirring for 2 d, the reaction was slowly quenched via portion-wise addition to rapidly stirring ice water (820 mL). The mixture was stirred at ambient temperature for 10 min and then vacuum filtered. Solids collected were rinsed with water and air-dried, yielding the title compounds as an orange solid in an isomeric ratio of about 4:1 (by 1H NMR) with the 6-Br isomer as the major isomer (21 g). The wet solid isomeric mixture (about 75% w/w) was directly used in Step 3 without further purification. MS (apci) m/z = 298.9, 300.9 (M+H). Regioisomeric ratio was determined by MeO chemical shift in 1H NMR (CDCl3) delta 3.98 (6-Br isomer) vs.3.83 (4-Br isomer). The isomeric mixture of ethyl-6-bromo-4- methoxypyrazolo[1,5-a]pyridine-3-carboxylate and ethyl-4-bromo-6-methoxypyrazolo[1,5- a]pyridine-3-carboxylate from Step 2 (15 g, 50.1 mmol) was added to 48% HBr (114 mL) while stirring, then heated at 80C for 90 min, followed by stirring at ambient temperature overnight. The resulting suspension was vacuum filtered and rinsed with water. The aqueous filtrate and the filter cake were treated independently. The filter cake was taken up in MTBE and vacuum filtered to remove insoluble impurities. The MTBE filtrate was dried over anhydrous Na2SO4, filtered and concentrated in vacuo to yield 6-bromo-4-methoxypyrazolo[1,5-a]pyridine as a beige solid (about 98:2 6-/4- Br; 5.08 g). MS (apci) m/z = 226.9, 228.9 (M+H). 1H NMR (CDCl3): delta 8.26 (m, 1H), 7.82 (d, 1H), 6.61 (m, 1H), 6.43 (m, 1H), 3.94 (s, 3H). Independently, the original aqueous reaction mixture filtrate was extracted with EtOAc (2 × 500 mL). The combined organic extracts were dried (Na2SO4), filtered and concentrated in vacuo. The crude residue was taken up in DCM (50 mL) and then filtered to remove insoluble solids. Concentration of the DCM filtrate under vacuum followed by silica chromatography (0 to 50% EtOAc/hexanes) yielded a second batch of 6-bromo- 4-methoxypyrazolo[1,5-a]pyridine (Intermediate 1) as a white solid (upper Rf spot, 2.06 g), as well as the minor isomer title compound 4-bromo-6-methoxypyrazolo[1,5-a]pyridine also as a white solid (lower Rf spot, 1.32 g). MS (apci) m/z = 226.9, 228.9 (M+H).1H NMR (CDCl3): delta 8.02 (m, 1H), 7.85 (d, 1H), 7.17 (d, 1H), 6.55 (m, 1H), 3.80 (s, 3H). |
| 1.32 g |
|
To a magnetically stirred white suspension of 1-amino-3-bromo-5-methoxypyridin-1-ium 2,4,6- trimethylbenzenesulfonate (33.24 g, 82.42 mmol) in DMF (82 mL) at ambient temperature was added TEA (22.98 mL, 164.8 mmol), followed by drop-wise addition of ethyl propiolate (16.71 mL, 164.8 mmol). After vigorous stirring for 2 d, the reaction was slowly quenched via portion- wise addition to rapidly stirring ice water (820 mL). The mixture was stirred at ambient temperature for 10 min and then vacuum filtered. Solids collected were rinsed with water and air- dried, yielding the title compounds as an orange solid in an isomeric ratio of about 4:1 (by 1H NMR) with the 6-Br isomer as the major isomer (21 g). The wet solid isomeric mixture (about 75% w/w) was directly used in Step 3 without further purification. MS (apci) m/z = 298.9, 300.9 (M+H). Regioisomeric ratio was determined by MeO chemical shift in 1H NMR (CDCl3) delta 3.98 (6-Br isomer) vs.3.83 (4-Br isomer).; The isomeric mixture of ethyl 6-bromo-4- methoxypyrazolo[1,5-a]pyridine-3-carboxylate and ethyl 4-bromo-4-methoxypyrazolo[1,5- a]pyridine-3-carboxylate from Step 2 (15 g, 50.1 mmol) was added to 48% HBr (114 mL) while stirring, then heated at 80C for 90 min followed by stirring at ambient temperature overnight. The resulting suspension was vacuum filtered and rinsed with water. The aqueous filtrate and the filter cake were treated independently. The filter cake was taken up in MTBE and vacuum filtered to remove insoluble impurities. The MTBE filtrate was dried over anhydrous Na2SO4, filtered and concentrated in vacuo to yield 6-bromo-4-methoxypyrazolo[1,5-a]pyridine (Intermediate A1) as a beige solid (about 98:26-/4- Br; 5.08 g). MS (apci) m/z = 226.9, 228.9 (M+H).1H NMR (CDCl3) delta 8.26 (m, 1H), 7.82 (d, 1H), 6.61 (m, 1H), 6.43 (m, 1H), 3.94 (s, 3H). (1120) [00593] Independently the original aqueous reaction mixture filtrate was extracted with EtOAc (2 × 500 mL). The combined organic extracts were dried (Na2SO4), filtered and concentrated in vacuo. The crude residue was taken up in DCM (50 mL) and then filtered to remove insoluble solids. Concentration of the DCM filtrate under vacuum followed by silica chromatography (0 to 50% EtOAc/hexanes) yielded a second batch of 6-bromo-4-methoxypyrazolo[1,5-a]pyridine (Intermediate A1) as white solid (upper Rf spot, 2.06 g), as well as the minor isomer title compound 4-bromo-6-methoxypyrazolo[1,5-a]pyridine (Intermediate A2) also as white solid (lower Rf spot, 1.32 g). MS (apci) m/z = 226.9, 228.9 (M+H). 1H NMR (CDCl3) delta 8.02 (m, 1H), 7.85 (d, 1H), 7.17 (d, 1H), 6.55 (m, 1H), 3.80 (s, 3H). |
| 2.06 g; 1.32 g |
|
To a magnetically stirred white suspension of1 -amino-3-bromo-5-methoxypyridin-1 -ium 2,4,6-trimethyl- benzenesulfonate (2a) (33.24 g, 82.42 mmol) in DMF (82 mL) at ambient temperature was added TEA (22.98 mL, 164.8 mmol), followed by drop-wise addition of ethyl propiolate (16.71 mL, 164.8 mmol). Afier vigorous stirring for 2 d, the reaction was slowly quenched via portion-wise addition to rapidly stirring ice water (820 mL). The mixture was stirred at ambient temperature for 10 mm and then vacuum filtered. Solids collected were rinsed with water and air-dried, yielding the title compounds as an orange solid in an isomeric ratio of about 4:1 (by ?H NMR) with compound 3a as the major isomer (21 g). The wet solid isomeric mixture (about 75% w/w) was directly used in the next step without further purification. MS (apci) mlz=298.9, 300.9 (M+H). Regioisomeric ratio was determined by MeO chemical shift in ?H NMR (CDC13) oe 3.98 (3a) vs. 3.83 (4a).The isomeric mixture of ethyl-6-bromo-4- methoxypyrazolo[1 ,S-a]pyridine-3-carboxylate (3a) and ethyl-4-bromo-4-methoxypyrazolo[1 ,5-a]pyridine-3-car- boxylate (4a) described above (15 g, 50.1 mmol) was added to 48% HEr (114 mE) while stirring, tben beated at 80 C. for 90 mm, followed by stirring at ambient temperature overnight. The resulting suspension was vacuum filtered and rinsed with water. The aqueous filtrate and the filter cake were treated independently. The filter cake was taken up in MTEE and vacuum filtered to remove insoluble impurities. The MTEE filtrate was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to yield 6-bromo-4- methoxypyrazolo[1 ,5-a]pyridine (5A) as a beige solid (about 98:2 6-14-Br; 5.08 g). MS (apci) mlz=226.9, 228.9 (M+H). ?H NMR (CDC13): oe 8.26 (m, 1H), 7.82 (d, 1H), 6.61 (m, 1H), 6.43 (m, 1H), 3.94 (s, 3H). Independently, the original aqueous reaction mixture filtrate was extracted with EtOAc (2x500 mE). The combined organic extracts were dried (Na2504), filtered, and concentrated in vacuo. The crude residue was taken up in DCM (50 mE) and then filtered to remove insoluble solids. Concentration of the DCM filtrate under vacuum followed by silica chromatography (0 to 50% EtOAc/hexanes) yielded a second batch of 6-bromo-4-methoxypyrazolo[ 1 ,5-a]pyridine (5A) as white solid (upper Rf spot, 2.06 g), as well as the minor isomer title compound 4-bromo-6-methoxypyrazolo[1 ,5-a]pyridine (SC), also as white solid (lower Rf spot, 1.32 g). MS (apci) mlz=226.9, 228.9 (M+H). ?H NMR (CDC13) oe 8.02 (m, 1H), 7.85 (d, 1H), 7.17 (d, 1H), 6.55 (m, 1H), 3.80 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping