Alternatived Products of [ 120161-06-0 ]
Product Details of [ 120161-06-0 ]
| CAS No. : | 120161-06-0 |
MDL No. : | MFCD08277409 |
| Formula : |
C11H13N3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
187.24
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 120161-06-0 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 120161-06-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 120161-06-0 ]
- 1
-
 [ 123-75-1 ]
[ 123-75-1 ]

-
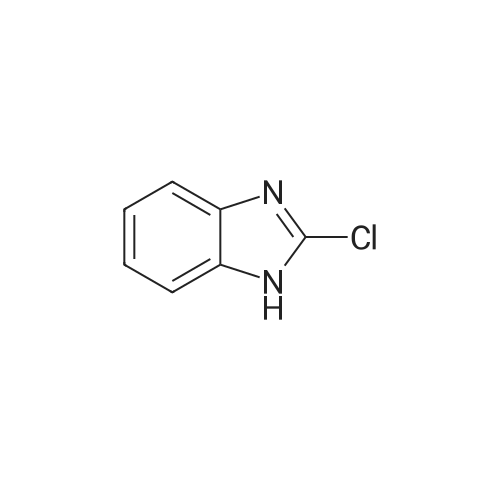 [ 4857-06-1 ]
[ 4857-06-1 ]

-
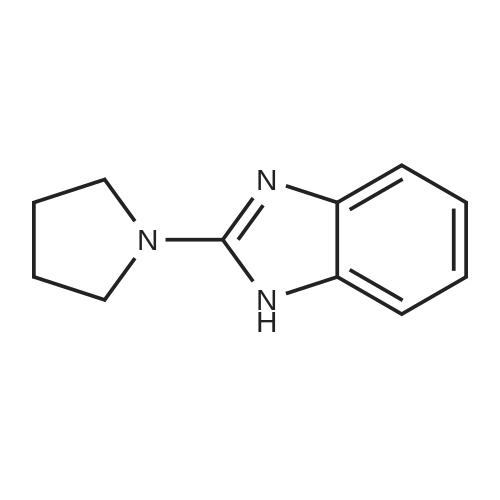 [ 120161-06-0 ]
[ 120161-06-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 41% |
In methanol at 160℃; for 2.5h; Microwave irradiation; |
3 Example 3: Preparation and structure identification of benzimidazole derivative TUP-01
Take 30 mL of the dried microwave tube, add 2-chlorobenzimidazole (0.457 g, 3 mmol), dissolve in 6 mL of methanol, and add tetrahydropyrrole (0.32 mL, 3.9 mmol).Microwave reaction at 160 ° C for 2.5 h. The solvent was dried and dissolved in 200 mL of ethyl acetate.It was washed with 200 mL of water and 200 mL of brine, and the organic phase was dried over anhydrous sodium sulfate. Spin the organic phase to obtain the benzimidazole derivative TUP-01,The white solid was 0.274 g, and the yield was 41%. |
|
In ethanol |
8.B Part B
Part B 2-Chloro-1H-benzimidazole (10.0 g, 0.066 mol), pyrrolidine (18.5 g, 0.26 mol), and ethanol (100 mL) were combined. The resulting solution was heated at 160-170° C. for 6 hours and then the solvent was evaporated. The resulting residue was mixed with water. The mixture was made strongly acidic with hydrochloric acid and then made basic with ammonium hydroxide. The resulting solid was isolated by filtration, washed with water and then air dried to provide 11.8 g of crude product as a tan powder. This material was recrystallized from ethyl acetate/methanol to provide 4.9 g of 2-pyrrolidino-1H-benzimidazole. Analysis: Calculated for C11H13N3: % C, 70.56; % H, 7.00; % N, 22.44. Found: % C, 70.13; % H, 7.05; % N, 22.70. |
|
In ethanol |
8.B Part B
Part B 2-Chloro-1H-benzimidazole (10.0 g, 0.066 mol), pyrrolidine (18.5 g, 0.26 mol), and ethanol (100 mL) were combined. The resulting solution was heated at 160-170° C. for 6 hours and then the solvent was evaporated. The resulting residue was mixed with water. The mixture was made strongly acidic with hydrochloric acid and then made basic with ammonium hydroxide. The resulting solid was isolated by filtration, washed with water and then air dried to provide 11.8 g of crude product as a tan powder. This material was recrystallized from ethyl acetate/methanol to provide 4.9 g of 2-pyrrolidino -1H-benzimidazole. Analysis: Calculated for C11H13N3: %C, 70.56; %H, 7.00; %N, 22.44; Found: %C, 70.13; %H, 7.05; %N, 22.70. |
|
at 200℃; for 0.5h; Microwave irradiation; |
|

Reference:
[1]Current Patent Assignee: TIBET UNIVERSITY - CN108997308, 2018, A
Location in patent: Paragraph 0049; 0050; 0051
[2]Current Patent Assignee: 3M CO - US6756382, 2004, B2
[3]Current Patent Assignee: 3M CO - US2003/144283, 2003, A1
[4]Asche, MacKenzie; Beninato, Madison; Gallegos, Wacey; Greka, Anna; Hinman, Jennifer; Hopkins, Corey R.; L. Pablo, Juan; Sharma, Swagat; Tolentino, Kirsten T.
[ChemMedChem, 2022]
- 2
-
 [ 123-75-1 ]
[ 123-75-1 ]

-
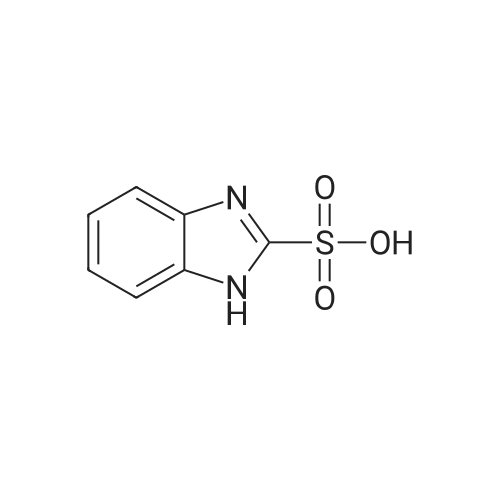 [ 40828-54-4 ]
[ 40828-54-4 ]

-
 [ 120161-06-0 ]
[ 120161-06-0 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







