| 88% |
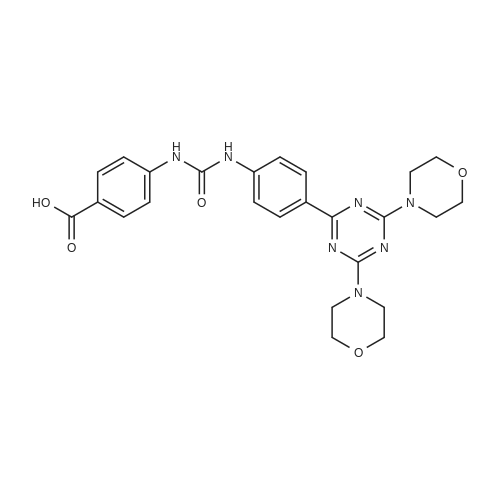
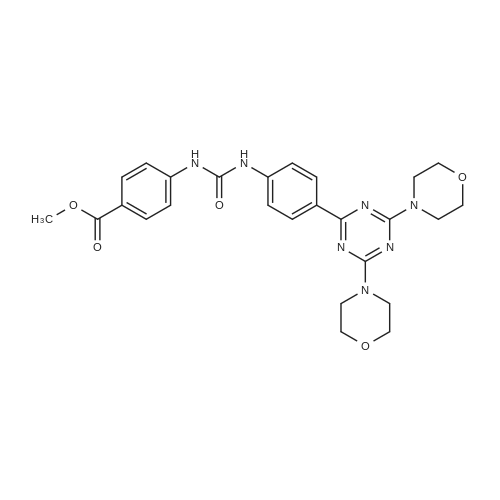
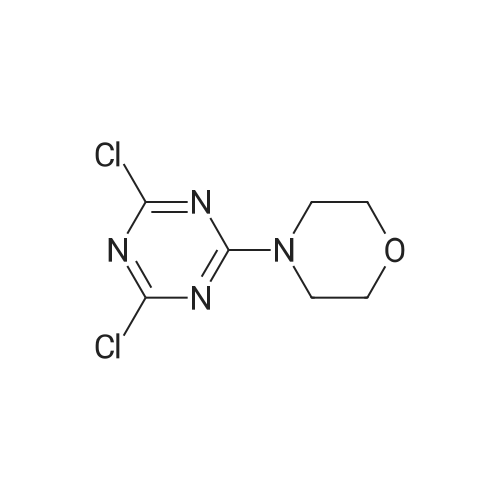
Stage #1: 4-(3-(4-(4,6-bismorpholine-1,3,5-triazin-2-yl)phenyl)ureido)benzoic acid With 1,1′-carbonyldiimidazole In tetrahydrofuran at 50℃; for 2h;
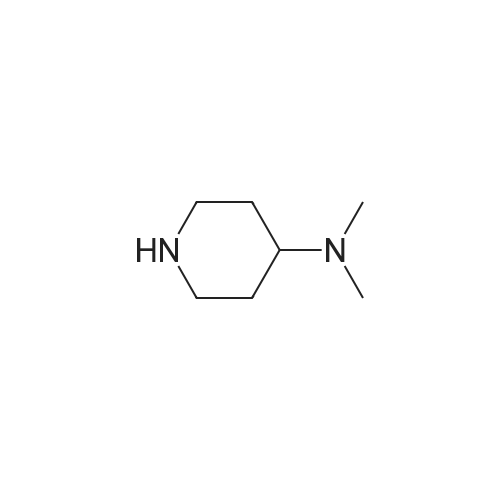
Stage #2: dimethyl-piperidin-4-yl-amine In tetrahydrofuran at 53℃; for 16h; |
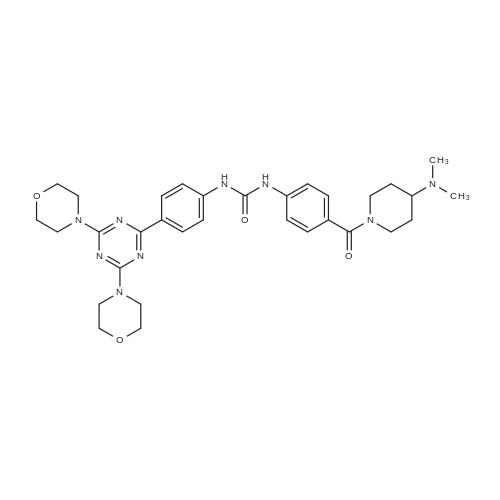
To a slurry of 4-(3-(4-(4,6-dimorpholin-4-yl-1,3,5-triazine-2-yl)phenyl)ureido)benzoic acid (7, 45.5 g, 0.09 mol) in dry THF (1.6 L) heated to 50 0C was added N,N'-carbonyl diimidazole (28 g, 0.17 mol). The reaction mixture was heated for 2 hours and followed by dimethylaminopiperidine (8, 23.5 g, 0.18 mol) and stirred at 53 0C for 16 hours. The reaction mixture was cooled to the room temperature and filtered. The cake was washed with 2-propanol and air-dried to give 97 % pure white powder in 88% yield (49.2 g, 0.08 mol). To the solids stirred in dimethyl acetamide (DMAC, 165 ml) at 70° C for 1 hour was added 2-propanol (640 ml) and the mixture was stirred at 65 0C for additional 1 hour. The solids were filtered, washed with 2-propanol and dried in a vacuum oven at 700C for 16 hour to give crystalline white powder (45 g) with >;99% purity. The above-mentioned work up process and crystallization procedure gave a Pd residue of 20 ppm. |
|
Stage #1: 4-(3-(4-(4,6-bismorpholine-1,3,5-triazin-2-yl)phenyl)ureido)benzoic acid With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.666667h; Inert atmosphere;
Stage #2: dimethyl-piperidin-4-yl-amine In N,N-dimethyl-formamide at 80℃; Inert atmosphere; |
4.1.1.5. Synthesis of compounds 6aa-ao, 6ba-bd, 6ca-cc and 6da-db.
General procedure: A mixture of 5 (0.4 mmol) in dry DMF (3 mL) was added DIPEA(0.8 mmol) and HBTU (0.48 mmol). The resulting mixture wasstirred at room temperature for 40 min under nitrogen atmosphere,and then, the corresponding secondary amine or amide(0.42 mmol) was added. The mixture was stirred under nitrogenatmosphere at 80 C for 8.0 h. Poured into ice-water, the mixturewas extract with DCM (50 mL 3). The combined the organicphases was washed with water (50 mL 3) and saturated NaClsolution (50 mL 1), and dried over Na2SO4. The solution wasconcentrated in vacuo and the crude product was purified by columnchromatography on silica gel (DCM: MeOH 10:1) to affordthe corresponding target compound in 40e90% yield.1-(4-(4-(Dimethylamino)piperidine-1-carbonyl)phenyl)-3-(4-(4,6-dimorpholino-1,3,5-triazin-2-yl)phenyl)urea (6aa).White solid;yield 59.9%; purity: 99.9%; Mp: 286e291 C. 1H NMR (600 MHz,DMSO-d6) d 9.06 (s, 1H),8.94 (s, 1H), 8.29 (d, J 8.8 Hz, 2H), 7.57 (d,J 8.8 Hz, 2H), 7.52 (d, J 8.6 Hz, 2H), 7.34 (d, J 8.5 Hz, 2H), 4.38(s, 1H), 3.82 (d, J 52.7 Hz, 9H), 3.66 (s, 8H), 2.90 (s, 2H), 2.33 (dd,J 12.4, 9.1 Hz, 1H), 2.17 (s, 6H), 1.75 (s, 2H), 1.39e1.26 (m, 2H); 13CNMR (150 MHz, DMSO-d6) d 169.4, 169.3, 165.1, 152.6, 143.2, 141.1,130.5, 129.9, 129.5, 128.5, 118.1, 117.8, 66.5, 61.8, 43.8, 41.9, 28.7.HRMS (ESI): m/z [MH] calcd. for [C32H42N9O4]: [C32H42N9O4]:616.3360, found: 616.3360. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping