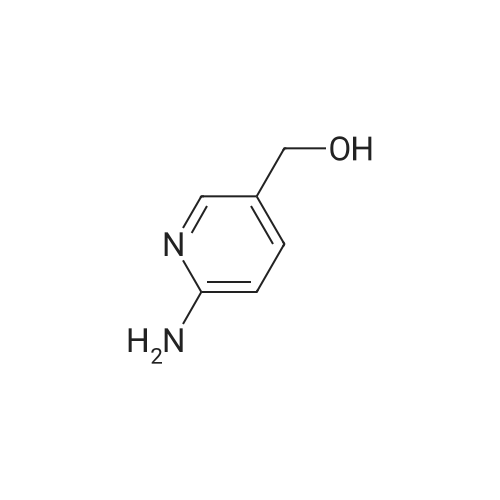
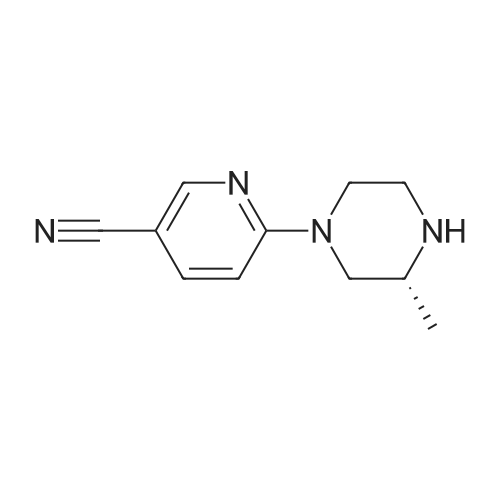
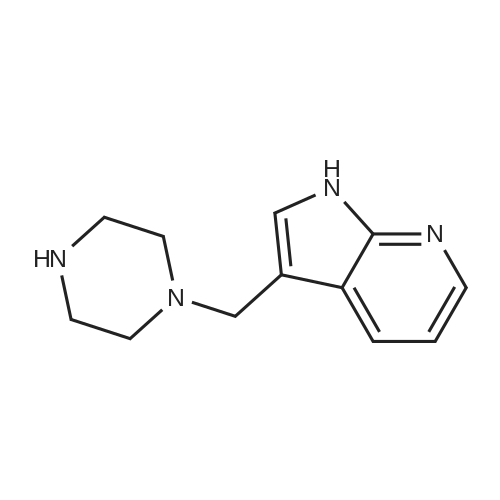
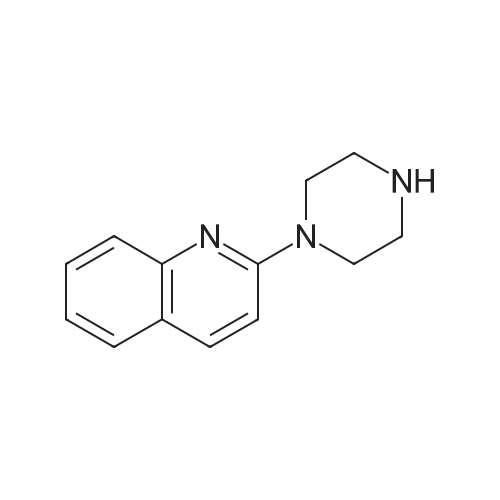
| 30% |
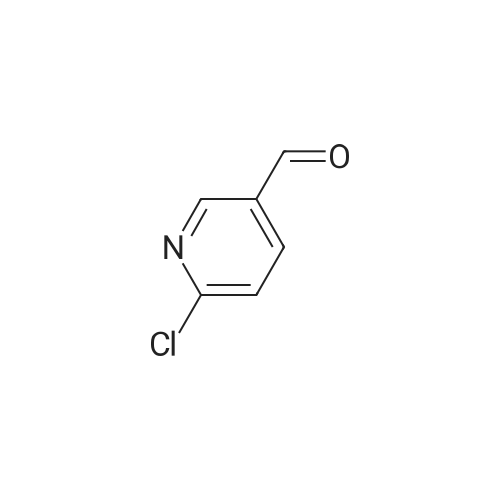
With potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; In 1,4-dioxane; at 150℃; for 1h; |
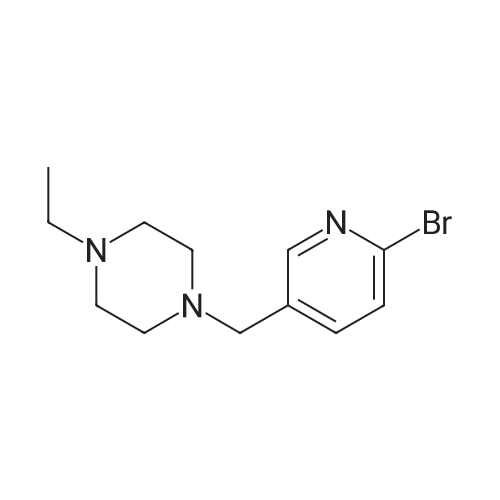
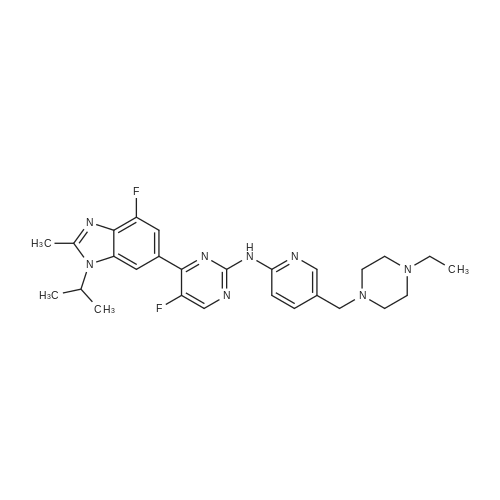
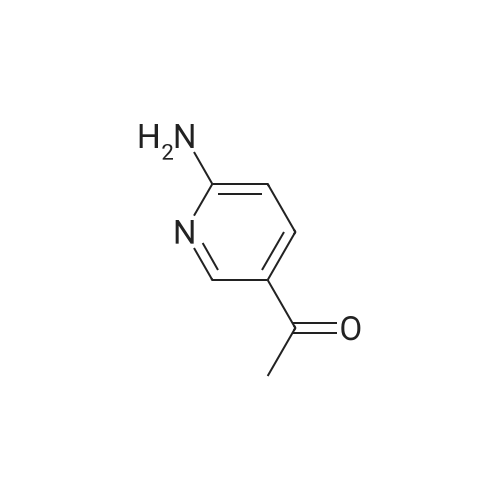
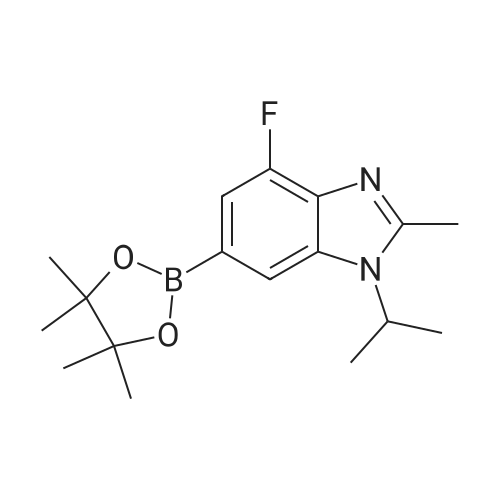
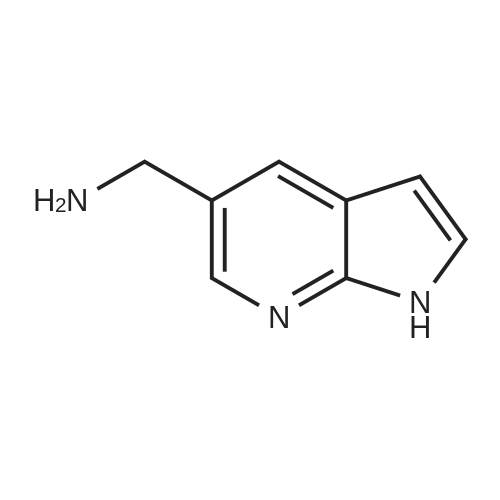
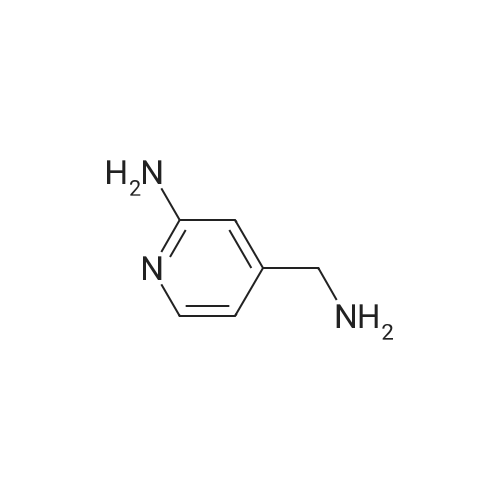
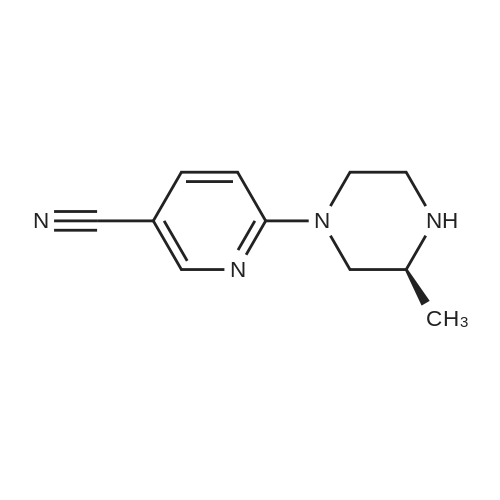
Pd2(dba)3 (86.4mg, 0.09mmol) and Xant-phos (109.2mg, 0.19mmol) were added under N2 to a solution of 154 1E (290.0mg, 0.94mmol), INT-7 (228.6mg, 1.04mmol), and 152 potassium phosphate (400.5mg, 1.88mmol) in 111 1,4-dioxane (10mL). Then the mixture was reacted in the microwave at 150C for 1h. The mixture was cooled to RT, filtered, diluted with water (10mL), and extracted with DCM (10mL×3). The combined organic layers were washed with brine (30mL), dried over anhydrous Na2SO4, concentrated under a vacuum, and purified by preparative thin-layer chromatography to obtain 157 compound 1 (140.3mg; yield, 30%) as a yellow solid. Purity: 99.6%; retention time: 3.10min. MP: 152.9-153.4C. High-resolution mass spectrometry (HRMS)-ESI: [M+H]+ observed 492.2677, calculated 492.2687.1H NMR (400MHz, CDCl3) delta 8.72 (s, 1H), 8.49 (d, 1H, J=3.2Hz), 8.38 (d, 1H, J=8.4Hz), 8.31 (s, 1H), 7.92 (s, 1H), 7.89 (s, 1H), 7.73 (d, 1H, J=8.4Hz), 3.52 (s, 2H), 2.54-2.41 (m, 10H), 2.38 (s, 3H), 1.40 (s, 6H), 1.10 (t, 3H, J=7.2Hz). 13C NMR (100MHz, CDCl3) delta 191.29, 155.37 (d, JC-F=3.0Hz), 154.76 (d, JC-F=252.0Hz), 152.17, 152.00, 150.94 (d, JC-F=9.0Hz), 149.63 (d, JC-F=89.0Hz), 149.37 (d, JC-F=3.0Hz), 147.39 (d, JC-F=26.0Hz), 142.82 (d, JC-F=11.0Hz), 139.05, 132.05 (d, JC-F=6.0Hz), 127.27, 117.97 (dd, JC-F=3.0Hz), 116.32 (dd, JC-F=7.0Hz), 111.39, 59.84, 54.85, 52.91 (2C), 52.74 (2C), 52.28, 23.03 (2C), 15.84, 11.95. |
| 140.3 mg |
With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; In 1,4-dioxane; at 150℃; for 1h;Microwave irradiation; Inert atmosphere; |
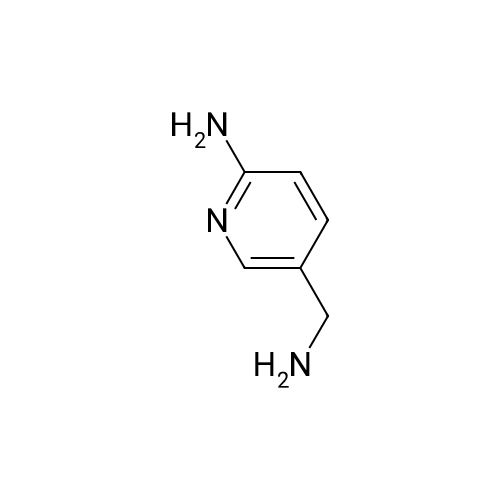
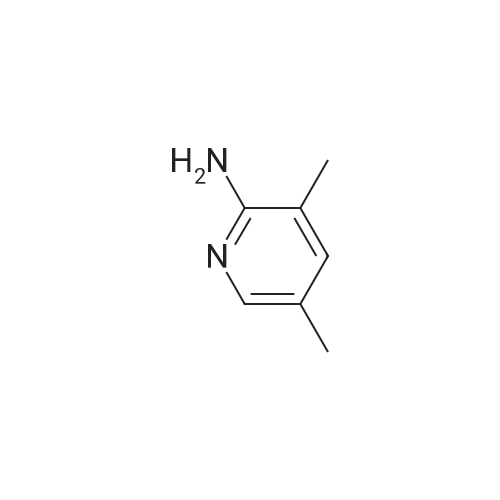
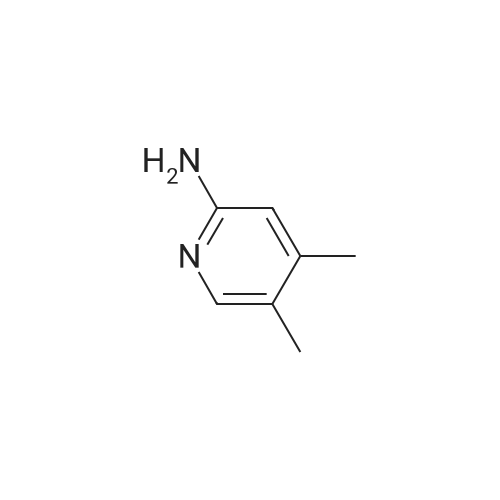
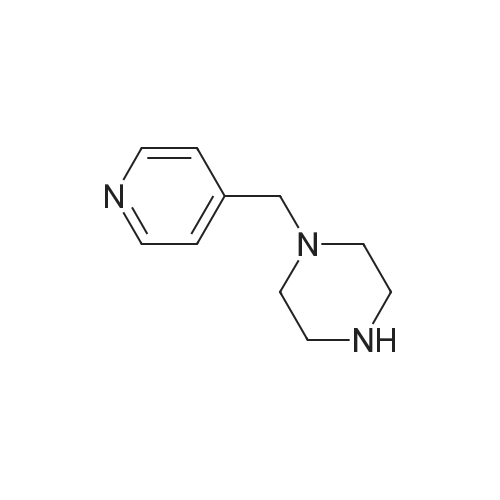
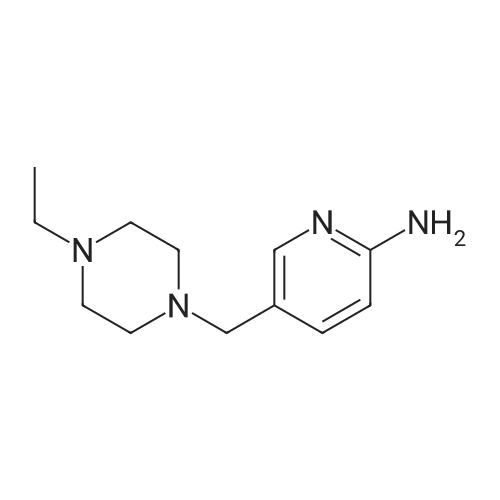
A reaction flask was charged with 5-(2-chloro-5-fluoropyrimidin-4-yl)-7-fluoro -2,3,3-trimethyl-3H-indole (290.0mg, 0.94 mmol) prepared in step 5, 5-((4-ethylpiperazin-1-yl)methyl)pyridin-2-amino (228.6 mg, 1.04 mmol) preparedin Step 2, potassium phosphate (400.5 mg, 1.88 mmol), 10 ml of dioxane, Pd2(dba)3 (86.4 mg, 0.09 mmol), and 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (109.2 mg, 0.19 mmol). Microwave reaction was carried out at 150C underthe protection of nitrogen gas for 1 h. The reaction product was cooled to room temperature, filtered, added with 10mlof water, extracted three times with dichloromethane (10 ml for each time). The organic phases were combined, washedonce with 30 ml of saturated salt solution, dried with anhydrous sodium sulfate, filtered, subjected to rotary evaporationto remove solvent, and separated by silica gel column chromatography (dichloromethane: methanol = 30:1) to give thetitled compound (140.3 mg, y ellow solid). 1H-NMR(400MHz,CDCl3) delta8.72(s,1H), 8.49(d,1H,J=3.2Hz), 8.38(d,1H,J=8.4Hz), 8.31(s,1H), 7.92(s,1H),7.89(s,1H), 7.73(d,1H,J=8.4Hz), 3.52(s,2H), 2.54-2.41(m,10H), 2.38(s,3H), 1.40(s,6H), 1.10(t,3H,J=7.2Hz). MS(ESI):m/z 492.2[M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping