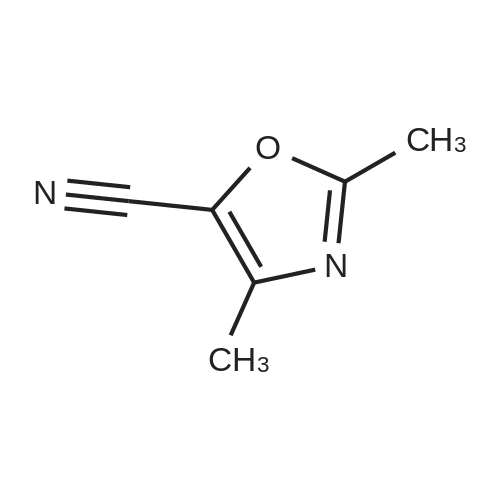
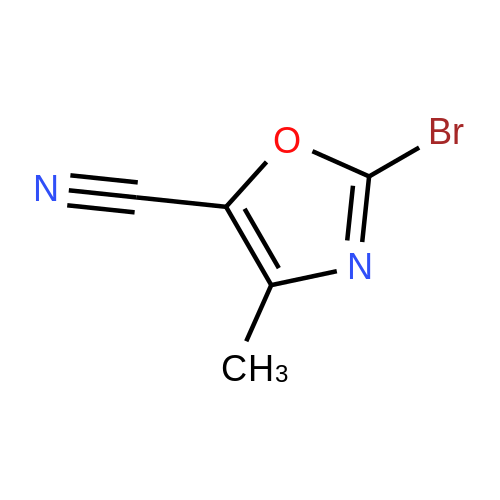
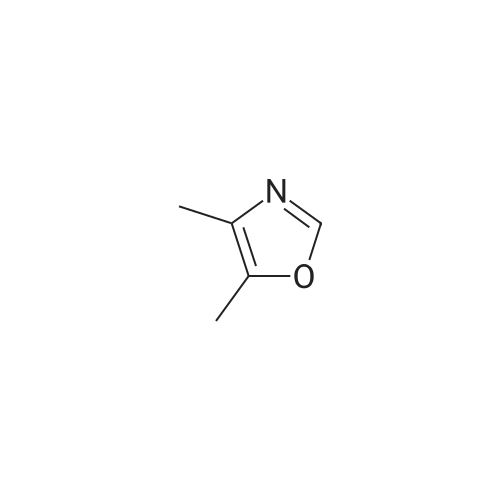
Alternatived Products of [ 1003-52-7 ]
Product Details of [ 1003-52-7 ]
| CAS No. : | 1003-52-7 |
MDL No. : | MFCD01111976 |
| Formula : |
C5H4N2O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | JZSLPQXOLZCAME-UHFFFAOYSA-N |
| M.W : |
108.10
|
Pubchem ID : | 70480 |
| Synonyms : |
|
Application In Synthesis of [ 1003-52-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1003-52-7 ]
- 1
-
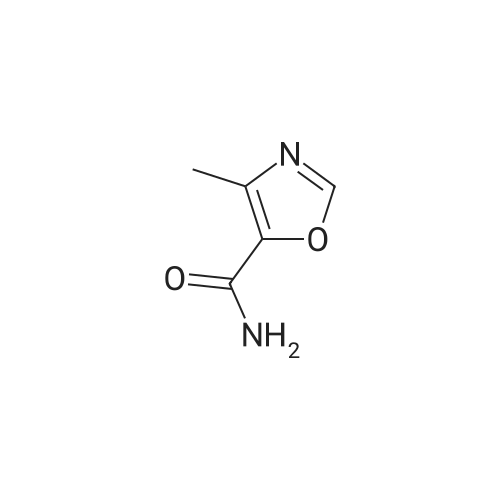 [ 4866-00-6 ]
[ 4866-00-6 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With phosphorus pentoxide |
|
|
With trichlorophosphate for 7h; Heating / reflux; |
110A
4-Methyl-oxazole-5-carboxylic acid amide (200 mg, 1.59 mmol) in POC13 (2 mL) was refluxed for 7 h. The reaction mixture was poured into water, and extracted with ethyl acetate (2X). The solvent was removed, and the resulting residue was chromatographed using hexane/ethyl acetate (3: 1) to give the title compound (240 mg). 'HNMR (500 MHz, CDC13) 5 ppm 2.41 (s, 3 H) 7.93 (s, 1H) |
- 2
-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
4-methyl-oxazole-5-carbothioic acid amide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogen sulfide In ethanol |
|
- 3
-
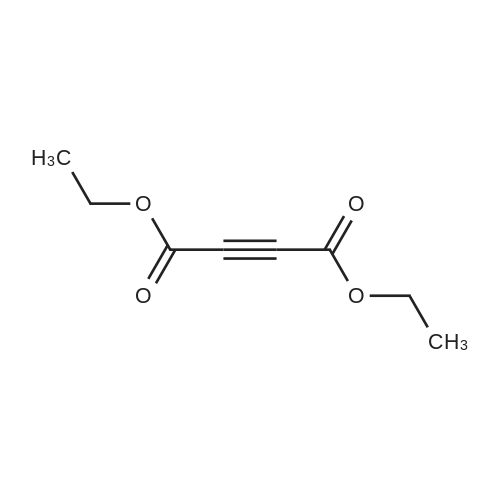 [ 762-21-0 ]
[ 762-21-0 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 30614-74-5 ]
[ 30614-74-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 75 % Turnov. |
With hydroquinone at 170℃; for 24h; |
|
- 4
-
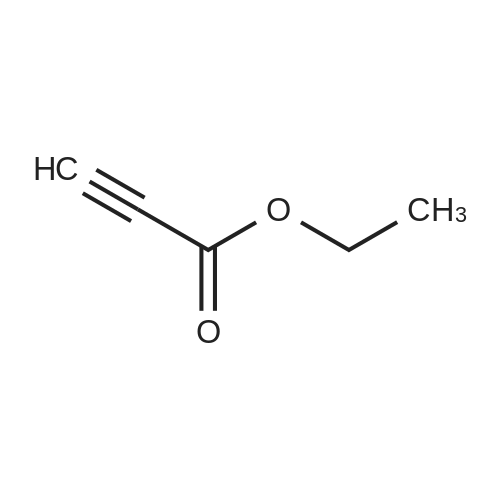 [ 623-47-2 ]
[ 623-47-2 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 80047-43-4 ]
[ 80047-43-4 ]

-
 [ 80047-42-3 ]
[ 80047-42-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 190℃; for 15h; Yield given. Yields of byproduct given; |
|
- 5
-
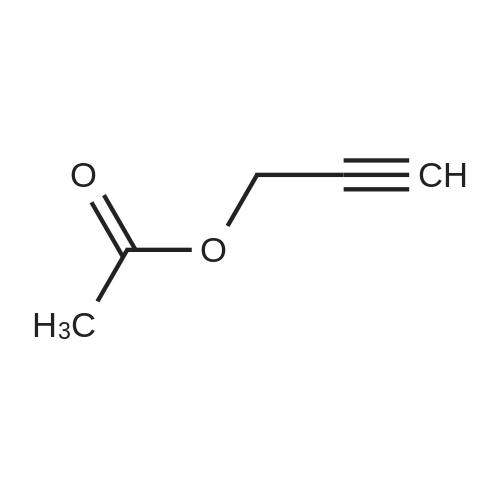 [ 627-09-8 ]
[ 627-09-8 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 30614-69-8 ]
[ 30614-69-8 ]

-
 [ 30610-18-5 ]
[ 30610-18-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydroquinone at 170℃; for 24h; Yield given; |
|
- 6
-
 [ 14700-53-9 ]
[ 14700-53-9 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 187467-53-4 ]
[ 187467-53-4 ]

-
 [ 187467-65-8 ]
[ 187467-65-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 4.4%
2: 20.1% |
With 2,6-di-tert-butyl-4-methyl-phenol; toluene-4-sulfonic acid for 15h; Heating; |
|
- 7
-
 [ 10192-66-2 ]
[ 10192-66-2 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 187467-61-4 ]
[ 187467-61-4 ]

-
3-[3-(1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridin-3-yl)-propyl]-1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 5.2%
2: 15.9% |
With acetic anhydride; hydroquinone for 15h; Heating; |
|
- 8
-
 [ 187467-53-4 ]
[ 187467-53-4 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 187467-65-8 ]
[ 187467-65-8 ]
| Yield | Reaction Conditions | Operation in experiment |
| 33.3% |
With 2,6-di-tert-butyl-4-methyl-phenol; toluene-4-sulfonic acid for 48h; Heating; |
|
- 9
-
 [ 10192-63-9 ]
[ 10192-63-9 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
7-(4,7-Dihydro-[1,3]dioxepin-2-ylmethyl)-3-methyl-5,9-dihydro-6,8-dioxa-2-aza-benzocyclohepten-4-ol
[ No CAS ]

-
3-(1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridin-3-yl-methyl)-1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 19.5%
2: 38.5% |
With acetic anhydride; hydroquinone for 15h; Heating; |
|
- 10
-
 [ 187467-61-4 ]
[ 187467-61-4 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
3-[3-(1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridin-3-yl)-propyl]-1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 15.8% |
With acetic anhydride; hydroquinone for 18h; Heating; |
|
- 11
-
7-(4,7-Dihydro-[1,3]dioxepin-2-ylmethyl)-3-methyl-5,9-dihydro-6,8-dioxa-2-aza-benzocyclohepten-4-ol
[ No CAS ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
3-(1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridin-3-yl-methyl)-1,5-dihydro-9-hydroxy-8-methyl-3H-[1,3]dioxepino[5,6-c]pyridine
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 15.2% |
With acetic anhydride; hydroquinone for 8h; Heating; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
Maleinsaeure-diaethylester/Essigsaeure -> 5-Hydroxy-6-methyl-pyridin-3.4-dicarbon- saeure-diaethylester, 5-Hydroxy-6-methyl-nicotinsaeure-aethylester; |
|
|
Fumarsaeure-diaethylester/Essigsaeure -> 5-Hydroxy-6-methyl-pyridin-3.4-dicarbon- saeure-diaethylester, 5-Hydroxy-6-methyl-nicotinsaeure-aethylester; |
|
|
Fumarsaeure-dinitril/Essigsaeure -> 5-Hydroxy-6-methyl-nicotinsaeure-nitril; |
|
|
Acrylsaeure-aethylester -> 5-Hydroxy-6-methyl-nicotinsaeure-aethylester; |
|

| Yield | Reaction Conditions | Operation in experiment |
| 99% |
|
4 EXAMPLE 4
EXAMPLE 4 A zirconium oxide produced from zirconyl chloride is used as the catalyst in the reactor described in Example 1. With a conversion of 99% the yield of 5-cyano-4-methyl-oxazole is 81%. |
| 99% |
|
8 EXAMPLE 8
EXAMPLE 8 A zirconium oxide catalyst produced from zirconium propylate is used in the reactor described in Example 1. With a conversion of 99% the yield of 5-cyano-4-methyl-oxazole is about 81%. |
| 98% |
|
5 EXAMPLE 5
EXAMPLE 5 A zirconium oxide catalyst coated on aluminum oxide is used in the reactor described in Example 1 and the temperature of the oven is adjusted to 320° C. With a conversion of 98% the yield of 5-cyano-4-methyl-oxazole is 76%. |
| 98% |
|
6 EXAMPLE 6
EXAMPLE 6 A hafnium oxide catalyst produced from hafnium oxychloride is used in the reactor described in Example 1. With a conversion of 98% the yield of 5-cyano-4-methyl-oxazole is 82%. |
| 96% |
|
3 EXAMPLE 3
EXAMPLE 3 A zirconium oxide produced from zirconium nitrate is used as the catalyst in the reactor described in Example 1. With a conversion of 96% the yield of 5-cyano-4-methyl-oxazole is 74%. |
|
aus d. entspr. Amid; |
|
|
5-Carbamoyl-4-methyloxazol, Chinolin, P2O5; |
|
|
4-Methyl-oxazol-5-carbonsaeure-amid , P2O5 oder DMF-SOCl2; |
|
|
4-Methyl-oxazol-5-carbonsaeure-amid , TiCl4, THF/Py.; |
|
|
|
3 EXAMPLE 3
EXAMPLE 3 63.1 g (0.5 mol, dried, with a maximum water content with 0.2%) of OXA were placed under argon in a 21 four-necked round flask fitted with a stirrer, thermometer, 100 ml dropping funnel (without pressure balance), three-way stopcock for inert gasification and Dimroth condenser. Subsequently, 285 ml (222.9 g, 5.43 mol) of acetonitrile and 350.2 ml (272.1 g, 1.47 mol) of tri(n-butyl) amine were added. The resulting suspension was stirred at 25° C. for 5 minutes and subsequently 40 ml (59.3 g, 0.35 mol) of SiCl4 were added dropwise from a dropping funnel within one hour in such a manner that the internal temperature in the flask did not rise above 45° C. After the addition of the SiCl4 the batch became reddish in colour. The reaction mixture was then heated to 55° C. in an oil bath, during which the content of the flask became dark in colour. The formation of OXN was determined directly from the batch by means of GC and addition of an internal standard (C11 -alkane). |
|
|
4 EXAMPLE 4
The working-up of the reaction product is effected analogously to Example 3. The yield of 5-cyano-4-methyl-oxazole amounts to 93%. |
|
Entspr. Amid, P4O10/Chinolin; |
|

- 14
-
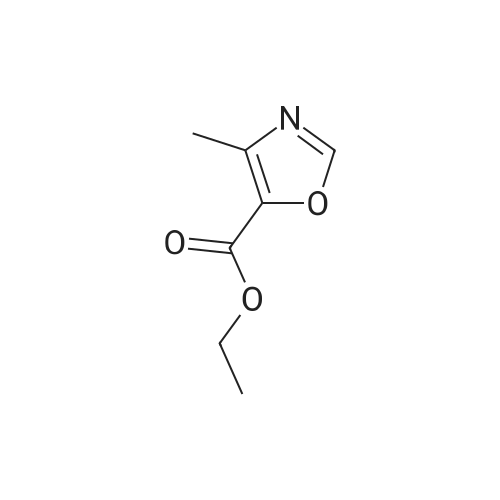 [ 20485-39-6 ]
[ 20485-39-6 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
- 15
-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 102170-37-6 ]
[ 102170-37-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: H2S / ethanol
2: acetic acid |
|
- 16
-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
4-methyl-5-(4-methyl-thiazol-2-yl)-oxazole
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: H2S / ethanol
2: acetic acid |
|
- 17
-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 106166-54-5 ]
[ 106166-54-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: H2S / ethanol
2: ethanol |
|
- 18
-
5-carbamoyl-4-methyl-oxazole (OXA)
[ No CAS ]

-
silicon tetrachloride (SiCl4)
[ No CAS ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]

-
 [ 4866-00-6 ]
[ 4866-00-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
1 General procedure for the dehydration of 5-carbamoyl-4-methyl-oxazole to 5-cyano-4-methyl-oxazole
EXAMPLE 1 General procedure for the dehydration of 5-carbamoyl-4-methyl-oxazole to 5-cyano-4-methyl-oxazole 2.52 g (20 mmol) of 5-carbamoyl-4-methyl-oxazole (OXA) were placed under argon in a 350 ml four-necked sulphonation flask fitted with a stirrer, cooled stirring jacket, thermometer, 50 ml dropping funnel (without pressure balance) and Dimroth condenser. Subsequently, 60-80 ml of a solvent and 60 mmol of an amine as the base were added. Thereafter, 22-60 mmol of silicon tetrachloride (SiCl4) were added dropwise as the dehydrating agent to the suspension present while stirring well in such a manner that the internal temperature in the flask did not exceed 45° C. The dropwise addition took about 15 minutes. The batch was subsequently heated to 60° C., becoming reddish to very dark brown in colour. The yield of 5-cyano-4-methyl-oxazole (OXN) was determined in each case by gas chromatography (GC) and the content determinations were effected in each case using an internal standard. |
- 19
-
 [ 872-50-4 ]
[ 872-50-4 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
3 EXAMPLE 3
In the distillative working-up of the reaction product there are obtained 592 g of N-methyl-pyrrolidone, thus a total of 2420 g (96.4%) of the N-methyl-pyrrolidone used. The amount of 5-cyano-4-methyl-oxazole obtained amounts to 222 g (93%). |
- 20
-
 [ 108-77-0 ]
[ 108-77-0 ]

-
 [ 4866-00-6 ]
[ 4866-00-6 ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In N,N-dimethyl-formamide |
EXAMPLE
EXAMPLE 22.88 g (198 mmol) of 5-carbamoyl-4-methyl-oxazole are suspended in 100 ml of N,N-dimethylformamide at room temperature and treated within 15 minutes with 18.31 g (99.3mmol) of cyanuric chloride in 250 ml of methyl tertiary butyl ether. The mixture is stirred at room temperature for one hour, whereby the initially yellow suspension becomes orange. Subsequently, it is neutralized with 50 ml of saturated aqueous Na2 CO3 solution, the phases are separated, the aqueous phase is extracted twice with 150 ml of methyl tertiary butyl ether each time and the combined organic phases are washed with 250 ml of dist. water, dried over Na2 SO4, filtered and concentrated on a rotary evaporator. An orange, liquid crude product remains. The yield of 5-cyano-4-methyl-oxazole is 99.4% of theory (determined by gas chromatography). |
- 21
-
2-amino-1-cyanopropenyl formate
[ No CAS ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 68 %Spectr. |
With acetic acid at 110℃; for 1h; |
6
To the solution of compound 1 prepared according to Example 1, 10 ml of acetic acid was added. The low-boiling solvent was removed by rotary evaporation, and the resulting acetic acid solution was heated to 110°C and stirred for 1 hour, to provide 4-methyl-oxazole-5-carbonitrile. Quantitative HNMR (CDCl3) indicated that the yield was 68%. |
|
With formic acid; acetic anhydride at 90℃; for 0.5h; |
1-6; 1-2 Example 1
Into a 20 mL round-bottomed flask, 2-amino-l-cyanopropenyl formate 1 (416 mg, purity 90%) was added in one portion to the solution of formic acid (2 mL) and acetic anhydride (1 mL) in propylene carbonate (5 ml) at 90°C. The reaction mixture was stirred at the same temperature for 30 mins and then cooled down to room temperature. The compound 2 was obtained and quantitative NMR indicated the yield was 91%. |
- 22
-
C5H6N2O
[ No CAS ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 65% |
Stage #1: C5H6N2O With boron trifluoride diethyl etherate In 1,2-dichloro-ethane Reflux;
Stage #2: With [bis(acetoxy)iodo]benzene In 1,2-dichloro-ethane Reflux; |
11 Example 11
To a solution of the compound 4 (220 mg, 2.0 mmol) in dried 1,2-dichloroethane (20 mL) was added BF3-Et20 (4.0 mmol, 2.0 eq). The reaction mixture was heated to reflux, and then phenyliodine(lll) diacetate (838 mg, 2.6 mmol, 1.3 eq) was added in one portion rapidly. After stirring under refluxing condition for 0.5-3 hours, the reaction mixture was cooled down to room temperature, quenched with saturated aqueous NaHC03, and then extracted with dichloromethane. The combined organic layer was washed with brine, dried over anhydrous lla2SC>4 and concentrated by the rotary evaporator. The crude product was purified by flash column chromatography to give the compound 5 (140 mg, 65% yield). |
- 23
-
C5H5BrN2O
[ No CAS ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 101 mg |
With zinc trifluoromethanesulfonate; 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 70℃; for 2h; Inert atmosphere; |
14-26 Example 23
Under N2 atmosphere, to a solution of compound 2 (191 mg, 1.01 mmol) and zinc triflate (38 mg, 0.1 mmol) in ACN (10 mL), DBU (387 ul, 2.60 mmol) was added. The reaction mixture was stirred at 70°C for two hours. After reaction completed, the reaction mixture was cooled to room temperature. The yield of compound 3 was determined by quantitative HPLC (101 mg, 93% yield). |
- 24
-
C11H11IN2O2
[ No CAS ]

-
 [ 1003-52-7 ]
[ 1003-52-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 80% |
Stage #1: C11H11IN2O2 With formic acid at 60℃; for 0.25h;
Stage #2: With formic acid; acetic anhydride at 90℃; for 2h; |
2-6 Example 2
Into a 100 mL round-bottomed flask, compound 1 (10 g) obtained according to Example 1 was added into the solution of formic acid (400 ul) in propylene carbonate (50 ml) at 60°C in one portion. The reaction mixture was cooled down to room temperature after stirred at 60°C for 15 min. The resulting solution was added dropwise to the solution of formic acid (20 ml) and acetic anhydride (3.8 ml) at 90°C. the reaction mixture was stirred at same temperature for additional 2 hours to afford 5-cyano-4-methyloxazole (2) in 80% yield |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping